Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer's disease
- PMID: 28117917
- PMCID: PMC5642282
- DOI: 10.1111/nan.12387
Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer's disease
Abstract
Aims: Hyperphosphorylated tau neuronal cytoplasmic inclusions (ht-NCI) are the best protein correlate of clinical decline in Alzheimer's disease (AD). Qualitative evidence identifies ht-NCI accumulating in the isodendritic core before the entorhinal cortex. Here, we used unbiased stereology to quantify ht-NCI burden in the locus coeruleus (LC) and dorsal raphe nucleus (DRN), aiming to characterize the impact of AD pathology in these nuclei with a focus on early stages.
Methods: We utilized unbiased stereology in a sample of 48 well-characterized subjects enriched for controls and early AD stages. ht-NCI counts were estimated in 60-μm-thick sections immunostained for p-tau throughout LC and DRN. Data were integrated with unbiased estimates of LC and DRN neuronal population for a subset of cases.
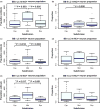
Results: In Braak stage 0, 7.9% and 2.6% of neurons in LC and DRN, respectively, harbour ht-NCIs. Although the number of ht-NCI+ neurons significantly increased by about 1.9× between Braak stages 0 to I in LC (P = 0.02), we failed to detect any significant difference between Braak stage I and II. Also, the number of ht-NCI+ neurons remained stable in DRN between all stages 0 and II. Finally, the differential susceptibility to tau inclusions among nuclear subdivisions was more notable in LC than in DRN.
Conclusions: LC and DRN neurons exhibited ht-NCI during AD precortical stages. The ht-NCI increases along AD progression on both nuclei, but quantitative changes in LC precede DRN changes.
Keywords: Alzheimer disease; Human; brain stem; dorsal raphe nucleus; locus coeruleus; unbiased stereology.
© 2017 British Neuropathological Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Korczyn AD. Why have we failed to cure Alzheimer’s disease? Journal of Alzheimer’s disease: JAD. 2012;29:275–82. - PubMed
-
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. - PMC - PubMed
-
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332–84. - PubMed
-
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. - PubMed
-
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL, 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–66. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

