Role of Intrinsic (Graft) Versus Extrinsic (Host) Factors in the Growth of Transplanted Organs Following Allogeneic and Xenogeneic Transplantation
- PMID: 28117931
- PMCID: PMC5489354
- DOI: 10.1111/ajt.14210
Role of Intrinsic (Graft) Versus Extrinsic (Host) Factors in the Growth of Transplanted Organs Following Allogeneic and Xenogeneic Transplantation
Abstract
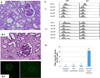
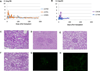
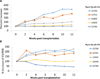
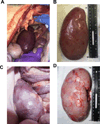
In our studies of life-supporting α-1,3-galactocyltransferase knockout (GalT-KO) pig-to-baboon kidneys, we found that some recipients developed increased serum creatinine with growth of the grafts, without histological or immunological evidence of rejection. We hypothesized that the rapid growth of orthotopic pig grafts in smaller baboon recipients may have led to deterioration of organ function. To test this hypothesis for both kidneys and lungs, we assessed whether the growth of outbred (Yorkshire) organ transplants in miniature swine was regulated by intrinsic (graft) or extrinsic (host environment) factors. Yorkshire kidneys exhibited persistent growth in miniature swine, reaching 3.7 times their initial volume over 3 mo versus 1.2 times for miniature swine kidneys over the same time period. Similar rapid early growth of lung allografts was observed and, in this case, led to organ dysfunction. For xenograft kidneys, a review of our results suggests that there is a threshold for kidney graft volume of 25 cm3 /kg of recipient body weight at which cortical ischemia is induced in transplanted GalT-KO kidneys in baboons. These results suggest that intrinsic factors are responsible, at least in part, for growth of donor organs and that this property should be taken into consideration for growth-curve-mismatched transplants, especially for life-supporting organs transplanted into a limited recipient space.
Keywords: growth and development; kidney (allograft) function/dysfunction; kidney transplantation/nephrology; lung failure/injury; lung transplantation/pulmonology; organ allocation; translational research/science; xenotransplantation.
© 2017 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures





References
-
- Klassen DK, Edwards LB, Stewart DE, Glazier AK, Orlowski JP, Berg CL. The OPTN Deceased Donor Potential Study: Implications for Policy and Practice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(6):1707–1714. - PubMed
-
- Sachs DH. The pig as a xenograft donor. Pathologie-biologie. 1994;42(3):217–219. - PubMed
-
- Sato M, Kagoshima A, Saitoh I, Inada E, Miyoshi K, Ohtsuka M, et al. Generation of alpha-1,3-Galactosyltransferase-Deficient Porcine Embryonic Fibroblasts by CRISPR/Cas9-Mediated Knock-in of a Small Mutated Sequence and a Targeted Toxin-Based Selection System. Reproduction in domestic animals = Zuchthygiene. 2015;50(5):872–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

