A plant protein signal sequence improved humoral immune response to HPV prophylactic and therapeutic DNA vaccines
- PMID: 28118086
- PMCID: PMC5328226
- DOI: 10.1080/21645515.2017.1264766
A plant protein signal sequence improved humoral immune response to HPV prophylactic and therapeutic DNA vaccines
Abstract
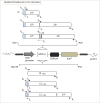
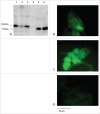
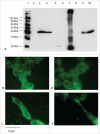
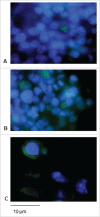
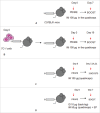
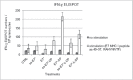
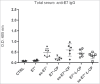
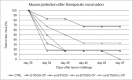
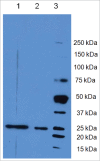
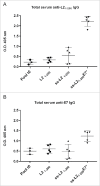
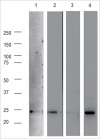
Signal sequences (ss) play a critical role in the sorting of nascent secretory and membrane proteins. This function has been conserved from bacteria through eukaryotes, although ss appear diverse in length and amino acid composition. Sorting of proteins is also critical to instruct antigens for a proper immunological response. Thus, a plant ss was used to drive Human Papillomavirus (HPV) model antigens into the human secretory pathway: the HPV16 E7 oncoprotein, its chimera with the coat protein (CP) of the Potato Virus X (PVX), the first 200 amino acids of the HPV16 minor capsid protein L2 (known to harbour cross-reacting epitopes) and its chimera with E7 gene. These genes were used to transfect HEK-293 cells and to immunize C57BL/6 mice. The ss-provided genes were expressed, and proteins detected by immunofluorescence and immunoblotting. Mouse immunization with DNA constructs carrying the ss elicited a strong humoral response against both E7 and L2 and a weak cell-mediated immunity. To our knowledge this is the first demonstration that a signal sequence derived from a plant can modulate the sorting of a heterologous protein in mammalian cells. This activity in mammalian cells may be responsible for the observed increased humoral response to DNA-based vaccines that are generally weak inducers of IgG response. This might open new perspectives in the design of DNA vaccines, especially to counteract infections where a strong humoral response is needed.
Keywords: HPV; humoral immune response; infectious diseases; plant signal sequences; vaccines.
Figures
References
-
- Vici P, Mariani L, Pizzuti L, Sergi D, Di Lauro L, Vizza E, Tomao F, Tomao S, Mancini E, Vincenzoni C, et al.. Emerging biological treatments for uterine cervical carcinoma. J Cancer 2014; 5:86-97; PMID:24494026; http://dx.doi.org/10.7150/jca.7963 - DOI - PMC - PubMed
-
- Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. J Transl Med 2010; 8:105; PMID:20979636; http://dx.doi.org/10.1186/1479-5876-8-105 - DOI - PMC - PubMed
-
- Tyler M, Tunban E, Chackerian B. Second-generation prophylactic HPV vaccines: successes and challenges. Expert Rev Vaccines 2014; 13:247-55; PMID:24350614; http://dx.doi.org/10.1586/14760584.2014.865523 - DOI - PMC - PubMed
-
- Ghambira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol 2007; 81:13927-31; PMID:17928339; http://dx.doi.org/10.1128/JVI.00936-07 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
