CD45RA Distinguishes CD4+CD25+CD127-/low TSDR Demethylated Regulatory T Cell Subpopulations With Differential Stability and Susceptibility to Tacrolimus-Mediated Inhibition of Suppression
- PMID: 28118317
- PMCID: PMC5265687
- DOI: 10.1097/TP.0000000000001278
CD45RA Distinguishes CD4+CD25+CD127-/low TSDR Demethylated Regulatory T Cell Subpopulations With Differential Stability and Susceptibility to Tacrolimus-Mediated Inhibition of Suppression
Abstract
Background: Adoptive transfer of forkhead box protein (FOX)3 regulatory T (Treg) cells offers a promising strategy to reduce damage to an allograft by the recipient's immune system. Identification of cell surface markers sufficient to purify Treg cells expanded ex vivo to remove cellular contaminants requires optimization. Furthermore, the expanded Treg must be able to survive, expand, and suppress in allograft recipients exposed to immunosuppressants, such as tacrolimus (TAC). Reduced CD127 expression enhances identification of Treg in the human CD4CD25 population. CD45RA expression identifies naive CD4CD25 Treg with an enhanced stability of Treg phenotype.
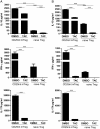
Methods: We combine an analysis of CD45RA, CD25, and CD127 expression to identify subpopulations of CD4CD127CD25 cells. Regulatory T cells were sorted according to expression of CD25 and CD45RA and expanded in the presence of a physiological relevant concentration of TAC. Regulatory T cell-specific demethylation region (TSDR) demethylation, FOXP3 expression, and suppression were analyzed.
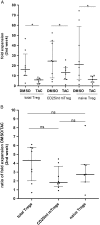
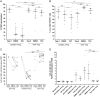
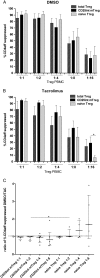
Results: CD4CD127CD25CD45RA Treg cells had a stable TSDR demethylated FOXP3 phenotype after expansion whereas CD4CD127CD25CD45RA Treg cell lost the TSDR demethylated phenotype. CD45RA Treg had a greater capacity to suppress after expansion with TAC.
Conclusions: Although CD45RA Treg retained a greater suppressive capacity when expanded with TAC, the marked loss of the TSDR demethylated status highlights the potential for loss of stability of these cells in transplant recipients treated with TAC based immunosuppression. We show that a population of CD4CD127CD45RA Regulatory T cell may offer the best compromise between susceptibility to loss of suppression when exposed to TAC and maintenance of a TSDR demethylated phenotype following in vitro expansion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–2243. - PubMed
-
- Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417–430. - PubMed
-
- van der Net JB, Bushell A, Wood KJ, et al. Regulatory T cells: first steps of clinical application in solid organ transplantation. Transpl Int. 2016;29:3–11. - PubMed
-
- Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15:283–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

