DNA Polymerases ImuC and DinB Are Involved in DNA Alkylation Damage Tolerance in Pseudomonas aeruginosa and Pseudomonas putida
- PMID: 28118378
- PMCID: PMC5261740
- DOI: 10.1371/journal.pone.0170719
DNA Polymerases ImuC and DinB Are Involved in DNA Alkylation Damage Tolerance in Pseudomonas aeruginosa and Pseudomonas putida
Abstract
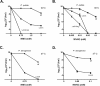
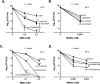
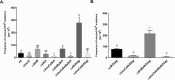
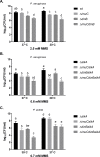
Translesion DNA synthesis (TLS), facilitated by low-fidelity polymerases, is an important DNA damage tolerance mechanism. Here, we investigated the role and biological function of TLS polymerase ImuC (former DnaE2), generally present in bacteria lacking DNA polymerase V, and TLS polymerase DinB in response to DNA alkylation damage in Pseudomonas aeruginosa and P. putida. We found that TLS DNA polymerases ImuC and DinB ensured a protective role against N- and O-methylation induced by N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) in both P. aeruginosa and P. putida. DinB also appeared to be important for the survival of P. aeruginosa and rapidly growing P. putida cells in the presence of methyl methanesulfonate (MMS). The role of ImuC in protection against MMS-induced damage was uncovered under DinB-deficient conditions. Apart from this, both ImuC and DinB were critical for the survival of bacteria with impaired base excision repair (BER) functions upon alkylation damage, lacking DNA glycosylases AlkA and/or Tag. Here, the increased sensitivity of imuCdinB double deficient strains in comparison to single mutants suggested that the specificity of alkylated DNA lesion bypass of DinB and ImuC might also be different. Moreover, our results demonstrated that mutagenesis induced by MMS in pseudomonads was largely ImuC-dependent. Unexpectedly, we discovered that the growth temperature of bacteria affected the efficiency of DinB and ImuC in ensuring cell survival upon alkylation damage. Taken together, the results of our study disclosed the involvement of ImuC in DNA alkylation damage tolerance, especially at low temperatures, and its possible contribution to the adaptation of pseudomonads upon DNA alkylation damage via increased mutagenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






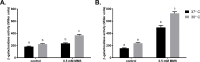
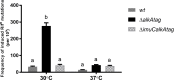

Similar articles
-
Pseudomonas putida AlkA and AlkB proteins comprise different defense systems for the repair of alkylation damage to DNA - in vivo, in vitro, and in silico studies.PLoS One. 2013 Oct 2;8(10):e76198. doi: 10.1371/journal.pone.0076198. eCollection 2013. PLoS One. 2013. PMID: 24098441 Free PMC article.
-
Oxidative DNA damage defense systems in avoidance of stationary-phase mutagenesis in Pseudomonas putida.J Bacteriol. 2007 Aug;189(15):5504-14. doi: 10.1128/JB.00518-07. Epub 2007 Jun 1. J Bacteriol. 2007. PMID: 17545288 Free PMC article.
-
DinB (DNA polymerase IV), ImuBC and RpoS contribute to the generation of ciprofloxacin-resistance mutations in Pseudomonas aeruginosa.Mutat Res. 2023 Jul-Dec;827:111836. doi: 10.1016/j.mrfmmm.2023.111836. Epub 2023 Aug 12. Mutat Res. 2023. PMID: 37625357
-
Mechanisms and consequences of methylating agent-induced SCEs and chromosomal aberrations: a long road traveled and still a far way to go.Cytogenet Genome Res. 2004;104(1-4):77-86. doi: 10.1159/000077469. Cytogenet Genome Res. 2004. PMID: 15162018 Review.
-
Methylating agents and DNA repair responses: Methylated bases and sources of strand breaks.Chem Res Toxicol. 2006 Dec;19(12):1580-94. doi: 10.1021/tx060164e. Chem Res Toxicol. 2006. PMID: 17173371 Free PMC article. Review.
Cited by
-
Snapshots of Pseudomonas aeruginosa SOS response reveal structural requisites for LexA autoproteolysis.iScience. 2025 Jan 2;28(2):111726. doi: 10.1016/j.isci.2024.111726. eCollection 2025 Feb 21. iScience. 2025. PMID: 39898034 Free PMC article.
-
Visualizing mutagenic repair: novel insights into bacterial translesion synthesis.FEMS Microbiol Rev. 2020 Sep 1;44(5):572-582. doi: 10.1093/femsre/fuaa023. FEMS Microbiol Rev. 2020. PMID: 32556198 Free PMC article. Review.
-
RecA and Specialized Error-Prone DNA Polymerases Are Not Required for Mutagenesis and Antibiotic Resistance Induced by Fluoroquinolones in Pseudomonas aeruginosa.Antibiotics (Basel). 2022 Feb 28;11(3):325. doi: 10.3390/antibiotics11030325. Antibiotics (Basel). 2022. PMID: 35326787 Free PMC article.
-
The Metabolic Redox Regime of Pseudomonas putida Tunes Its Evolvability toward Novel Xenobiotic Substrates.mBio. 2018 Aug 28;9(4):e01512-18. doi: 10.1128/mBio.01512-18. mBio. 2018. PMID: 30154264 Free PMC article.
-
Contribution of increased mutagenesis to the evolution of pollutants-degrading indigenous bacteria.PLoS One. 2017 Aug 4;12(8):e0182484. doi: 10.1371/journal.pone.0182484. eCollection 2017. PLoS One. 2017. PMID: 28777807 Free PMC article.
References
-
- Beranek DT. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res. 1990;231: 11–30. - PubMed
-
- Larson K, Sahm J, Shenkar R, Strauss B. Methylation-induced blocks to in vitro DNA replication. Mutat Res. 1985;150: 77–84. - PubMed
-
- Drabløs F, Feyzi E, Aas PA, Vaagbø CB, Kavli B, Bratlie MS, et al. Alkylation damage in DNA and RNA—repair mechanisms and medical significance. DNA Repair (Amst). 2004;3: 13891407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

