Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: A Fifty-Two-Week Randomized, Double-Blind, Placebo-Controlled Study
- PMID: 28118533
- PMCID: PMC5434872
- DOI: 10.1002/art.40049
Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: A Fifty-Two-Week Randomized, Double-Blind, Placebo-Controlled Study
Abstract
Objective: To assess the efficacy and safety of subcutaneous (SC) belimumab in patients with systemic lupus erythematosus (SLE).
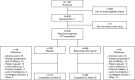
Methods: Patients with moderate-to-severe SLE (score of ≥8 on the Safety of Estrogens in Lupus Erythematosus National Assessment [SELENA] version of the SLE Disease Activity Index [SLEDAI]) were randomized 2:1 to receive weekly SC belimumab 200 mg or placebo by prefilled syringe in addition to standard SLE therapy for 52 weeks. The primary end point was the SLE Responder Index (SRI4) at week 52. Secondary end points were reduction in the corticosteroid dosage and time to severe flare. Safety was assessed according to the adverse events (AEs) reported and the laboratory test results.
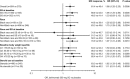
Results: Of 839 patients randomized, 836 (556 in the belimumab group and 280 in the placebo group) received treatment. A total of 159 patients withdrew before the end of the study. At entry, mean SELENA-SLEDAI scores were 10.5 in the belimumab group and 10.3 in the placebo group. More patients who received belimumab were SRI4 responders than those who received placebo (61.4% versus 48.4%; odds ratio [OR] 1.68 [95% confidence interval (95% CI) 1.25-2.25]; P = 0.0006). In the belimumab group, both time to and risk of severe flare were improved (median 171.0 days versus 118.0 days; hazard ratio 0.51 [95% CI 0.35-0.74]; P = 0.0004), and more patients were able to reduce their corticosteroid dosage by ≥25% (to ≤7.5 mg/day) during weeks 40-52 (18.2% versus 11.9%; OR 1.65 [95% CI 0.95-2.84]; P = 0.0732), compared with placebo. AE incidence was comparable between treatment groups; serious AEs were reported by 10.8% of patients taking belimumab and 15.7% of those taking placebo. A worsening of IgG hypoglobulinemia by ≥2 grades occurred in 0.9% of patients taking belimumab and 1.4% of those taking placebo.
Conclusion: In patients with moderate-to-severe SLE, weekly SC doses of belimumab 200 mg plus standard SLE therapy significantly improved their SRI4 response, decreased severe disease flares as compared with placebo, and had a safety profile similar to placebo plus standard SLE therapy.
Trial registration: ClinicalTrials.gov NCT01484496.
© 2017 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures


References
-
- Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999;285:260–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

