Brief Report: Pharmacodynamics, Safety, and Clinical Efficacy of AMG 811, a Human Anti-Interferon-γ Antibody, in Patients With Discoid Lupus Erythematosus
- PMID: 28118537
- PMCID: PMC5434930
- DOI: 10.1002/art.40052
Brief Report: Pharmacodynamics, Safety, and Clinical Efficacy of AMG 811, a Human Anti-Interferon-γ Antibody, in Patients With Discoid Lupus Erythematosus
Abstract
Objective: Interferon-γ (IFNγ) is implicated in the pathogenesis of discoid lupus erythematosus (DLE). This study sought to evaluate a single dose of AMG 811, an anti-IFNγ antibody, in patients with DLE.
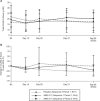
Methods: The study was designed as a phase I randomized, double-blind, placebo-controlled crossover study of the pharmacodynamics, safety, and clinical efficacy of AMG 811 in patients with DLE. Patients received a single subcutaneous dose of AMG 811 (180 mg) or placebo. The patients in sequence 1 received AMG 811 followed by placebo, while those in sequence 2 received placebo followed by AMG 811. Pharmacodynamic end points included global transcriptional analyses of lesional and nonlesional skin, IFNγ blockade signature (IGBS) transcriptional scores in the skin and blood, keratinocyte IFNγ RNA scores, and serum levels of CXCL10 protein. Additional end points were efficacy outcome measures, including the Cutaneous Lupus Erythematosus Disease Area and Severity Index, and safety outcome measures.
Results: Sixteen patients with DLE were enrolled in the study (9 in sequence 1 and 7 in sequence 2). AMG 811 treatment reduced the IGBS score (which was elevated in DLE patients at baseline) in both the blood and lesional skin. The keratinocyte IFNγ RNA score was not affected by administration of AMG 811. Serum CXCL10 protein levels (which were elevated in the blood of DLE patients) were reduced with AMG 811 treatment. The AMG 811 treatment was well tolerated but did not lead to statistically significant improvements in any of the efficacy outcome measures.
Conclusion: AMG 811 treatment led to changes in IFNγ-associated biomarkers and was well tolerated, but no significant clinical benefit was observed in patients with DLE.
Trial registration: ClinicalTrials.gov NCT01164917.
© 2017 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures

References
-
- Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981;4:471–5. - PubMed
-
- Fabbri P, Cardinali C, Giomi B, Caproni M. Cutaneous lupus erythematosus: diagnosis and management. Am J Clin Dermatol 2003;4:449–65. - PubMed
-
- Toro JR, Finlay D, Dou X, Zheng SC, LeBoit PE, Connolly MK. Detection of type 1 cytokines in discoid lupus erythematosus. Arch Dermatol 2000;136:1497–501. - PubMed
-
- Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci 2010;59:73–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

