Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children
- PMID: 28118559
- PMCID: PMC5310766
- DOI: 10.1056/NEJMoa1610493
Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children
Abstract
Background: Targeted temperature management is recommended for comatose adults and children after out-of-hospital cardiac arrest; however, data on temperature management after in-hospital cardiac arrest are limited.
Methods: In a trial conducted at 37 children's hospitals, we compared two temperature interventions in children who had had in-hospital cardiac arrest. Within 6 hours after the return of circulation, comatose children older than 48 hours and younger than 18 years of age were randomly assigned to therapeutic hypothermia (target temperature, 33.0°C) or therapeutic normothermia (target temperature, 36.8°C). The primary efficacy outcome, survival at 12 months after cardiac arrest with a score of 70 or higher on the Vineland Adaptive Behavior Scales, second edition (VABS-II, on which scores range from 20 to 160, with higher scores indicating better function), was evaluated among patients who had had a VABS-II score of at least 70 before the cardiac arrest.
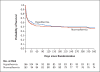
Results: The trial was terminated because of futility after 329 patients had undergone randomization. Among the 257 patients who had a VABS-II score of at least 70 before cardiac arrest and who could be evaluated, the rate of the primary efficacy outcome did not differ significantly between the hypothermia group and the normothermia group (36% [48 of 133 patients] and 39% [48 of 124 patients], respectively; relative risk, 0.92; 95% confidence interval [CI], 0.67 to 1.27; P=0.63). Among 317 patients who could be evaluated for change in neurobehavioral function, the change in VABS-II score from baseline to 12 months did not differ significantly between the groups (P=0.70). Among 327 patients who could be evaluated for 1-year survival, the rate of 1-year survival did not differ significantly between the hypothermia group and the normothermia group (49% [81 of 166 patients] and 46% [74 of 161 patients], respectively; relative risk, 1.07; 95% CI, 0.85 to 1.34; P=0.56). The incidences of blood-product use, infection, and serious adverse events, as well as 28-day mortality, did not differ significantly between groups.
Conclusions: Among comatose children who survived in-hospital cardiac arrest, therapeutic hypothermia, as compared with therapeutic normothermia, did not confer a significant benefit in survival with a favorable functional outcome at 1 year. (Funded by the National Heart, Lung, and Blood Institute; THAPCA-IH ClinicalTrials.gov number, NCT00880087 .).
Figures
Comment in
-
Cardiac resuscitation: Hypothermia or normothermia?Nat Rev Cardiol. 2017 Feb 14;14(3):130. doi: 10.1038/nrcardio.2017.17. Nat Rev Cardiol. 2017. PMID: 28195214 No abstract available.
-
Hypothermia after In-Hospital Cardiac Arrest in Children.N Engl J Med. 2017 Apr 27;376(17):1695-6. doi: 10.1056/NEJMc1702364. N Engl J Med. 2017. PMID: 28445674 No abstract available.
-
Hypothermia after In-Hospital Cardiac Arrest in Children.N Engl J Med. 2017 Apr 27;376(17):1695. doi: 10.1056/NEJMc1702364. N Engl J Med. 2017. PMID: 28447772 No abstract available.
-
Hypothermia after In-Hospital Cardiac Arrest in Children.N Engl J Med. 2017 Apr 27;376(17):1695. doi: 10.1056/NEJMc1702364. N Engl J Med. 2017. PMID: 28447773 No abstract available.
References
-
- Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication — a consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–83. - PubMed
-
- The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. - PubMed
-
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. - PubMed
-
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–206. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- R21 HD044955/HD/NICHD NIH HHS/United States
- U54 HD079123/HD/NICHD NIH HHS/United States
- R34 HD050531/HD/NICHD NIH HHS/United States
- U10 HD049945/HD/NICHD NIH HHS/United States
- P30 HD040677/HD/NICHD NIH HHS/United States
- CS-2015-15-016/DH_/Department of Health/United Kingdom
- U10 HD050096/HD/NICHD NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- U01 HD049934/HD/NICHD NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01 HL094339/HL/NHLBI NIH HHS/United States
- U10 HD050012/HD/NICHD NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- U10 HL109741/HL/NHLBI NIH HHS/United States
- UG1 HD050096/HD/NICHD NIH HHS/United States
- K23 NS075363/NS/NINDS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- U54 HD087011/HD/NICHD NIH HHS/United States
- U10 HD049981/HD/NICHD NIH HHS/United States
- U01 HL094345/HL/NHLBI NIH HHS/United States
- U10 HD049983/HD/NICHD NIH HHS/United States
- RL1 HD107773/HD/NICHD NIH HHS/United States
- U10 HD050009/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
