The involvement of endoplasmic reticulum stress response in immune dysfunction of dendritic cells after severe thermal injury in mice
- PMID: 28118617
- PMCID: PMC5354713
- DOI: 10.18632/oncotarget.14764
The involvement of endoplasmic reticulum stress response in immune dysfunction of dendritic cells after severe thermal injury in mice
Abstract
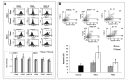
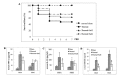
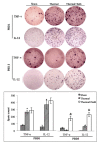
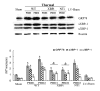
Suppressed adaptive immune function is one of the major concerns responsible for the development of opportunistic infections and subsequent sepsis with high mortality in severe burns. Endoplasmic reticulum stress (ERS) is the endogenous self-protective mechanism, and it plays an important role in almost every process of living by regulating the balance between homeostasis and apoptosis. The current study investigated the involvement of ERS in the pathogenesis of dysfunction of dendritic cells (DCs) in burn mice. Our results show a significant ERS response in splenic DC after burn injury. Treatment with salubrinal (Sal, reported to protect cells against ERS-induced apoptosis.) decrease the apoptotic rate of DC induced by burns, and promote maturation and activation of DC, as well as the ability to promote T cell proliferation and polarization towards Th1 immunity (all P<0.05). Gene silence of XBP-1 (key molecular in ERS response) results in the increased apoptosis and suppressed phenotypical maturation of splenic DC in burn mice. These results show that the excessive ERS is essential for immunosuppression during severe thermal injury. XBP-1 plays a pivotal role in DC functional immunomodulation in burn mice. Inhibition of apoptotic ERS response benefits mice from major burns.
Keywords: Pathology Section; burns; dendritic cell; endoplasmic reticulum stress; immune dysfunction; sepsis.
Conflict of interest statement
No authors have any financial interests to disclose.
Figures









References
-
- Jeschke MG, Pinto R, Kraft R, Nathens AB, Finnerty CC, Gamelli RL, Gibran NS, Klein MB, Arnoldo BD, Tompkins RG, Herndon DN. Inflammation and the Host Response to Injury Collaborative Research Program. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. 2015;43(4):808–15. doi: 10.1097/CCM.0000000000000790. - DOI - PMC - PubMed
-
- O’Sullivan ST. O’Connor TP. Immunosuppression following thermal injury: the pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50(8):615–23. - PubMed
-
- Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11(3):153–9. - PubMed
-
- Barlow Y. T lymphocytes and immunosuppression in the patient: a review. Burns. 1994;20(6):487–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

