Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine iv-busulfan myeloablative regimen: a report from the EBMT Acute Leukemia Working Party
- PMID: 28118857
- PMCID: PMC5259921
- DOI: 10.1186/s13045-016-0389-4
Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine iv-busulfan myeloablative regimen: a report from the EBMT Acute Leukemia Working Party
Abstract
Background: The impact of the use of anti-thymocyte globulin (ATG) in allogeneic stem cell transplantation performed with HLA-identical sibling donors following fludarabine and 4 days intravenous busulfan myeloablative conditioning regimen has been poorly explored.
Methods: We retrospectively analyzed 566 patients who underwent a first HLA-identical allogeneic stem cell transplantation with this conditioning regimen for acute myeloid leukemia in first complete remission between 2006 and 2013 and compared the outcomes of 145 (25.6%) patients who received ATG (ATG group) to 421 (74.4%) who did not (no-ATG group). The Kaplan-Meier estimator, the cumulative incidence function, and Cox proportional hazards regression models were used where appropriate.
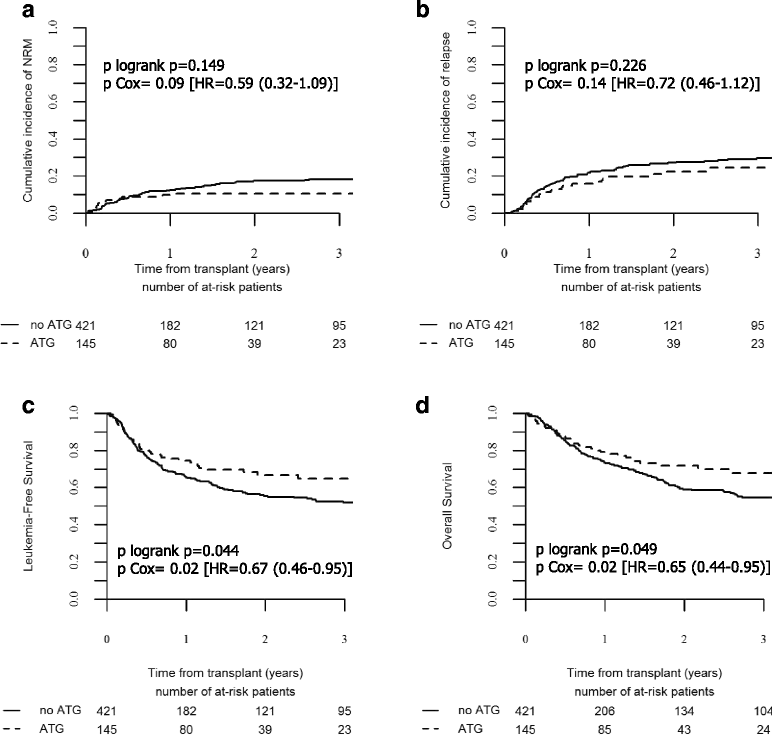
Results: Patients in the ATG group were older, received more frequently peripheral blood stem cell grafts from older donors, and were transplanted more recently. With a median follow-up of 19 months, patients in the ATG group had reduced 2-year cumulative incidence of chronic graft-versus-host disease (GVHD) (31 vs. 52%, p = 0.0002) and of its extensive form (8 vs. 26%, p < 0.0001) but similar relapse incidence (22 vs. 27%, p = 0.23) leading to improved GVHD and relapse-free survival (GRFS) (60 vs. 40%, p = 0.0001). In multivariate analyses, the addition of ATG was independently associated with lower chronic GVHD (HR = 0.46, p = 0.0001), improved leukemia-free survival (HR = 0.67, p = 0.027), overall survival (HR = 0.65, p = 0.027), and GRFS (HR = 0.51, p = 4 × 10-5). Recipient age above 50 years was the only other factor associated with worse survivals.
Conclusions: These results suggest that the use of ATG with fludarabine and 4 days intravenous busulfan followed by HLA-identical sibling donor allogeneic stem cell transplantation for acute myeloid leukemia improves overall transplant outcomes due to reduced incidence of chronic GVHD without increased relapse risk.
Keywords: Acute myeloid leukemia; Allogeneic stem cell transplantation; GRFS; Graft-versus-host disease; HLA-matched related donor; In vivo T cell depletion; Relapse incidence.
Figures


Similar articles
-
Reduced-Intensity Conditioning with Busulfan, Fludarabine, and Antithymocyte Globulin for Hematopoietic Cell Transplantation from Unrelated or Haploidentical Family Donors in Patients with Acute Myeloid Leukemia in Remission.Biol Blood Marrow Transplant. 2017 Sep;23(9):1555-1566. doi: 10.1016/j.bbmt.2017.05.025. Epub 2017 May 25. Biol Blood Marrow Transplant. 2017. PMID: 28552421 Clinical Trial.
-
Does anti-thymocyte globulin have a place in busulfan/fludarabine conditioning for matched related donor hematopoietic stem cell transplantation?Korean J Intern Med. 2016 Jul;31(4):750-61. doi: 10.3904/kjim.2015.234. Epub 2016 Mar 28. Korean J Intern Med. 2016. PMID: 27017944 Free PMC article.
-
Impact of Thymoglobulin by Stem Cell Source (Peripheral Blood Stem Cell or Bone Marrow) After Myeloablative Stem Cell Transplantation From HLA 10/10-Matched Unrelated Donors: A Report From the Société Française de Greffe de Moelle et de Thérapie Cellulaire.Transplantation. 2016 Aug;100(8):1732-9. doi: 10.1097/TP.0000000000000976. Transplantation. 2016. PMID: 26528768
-
Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning.Leukemia. 2003 May;17(5):841-8. doi: 10.1038/sj.leu.2402905. Leukemia. 2003. PMID: 12750695 Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
Cited by
-
[Chinese consensus of allogeneic hematopoietic stem cell transplantation for hematological disease (Ⅲ) -acute graft-versus-host disease (2020)].Zhonghua Xue Ye Xue Za Zhi. 2020 Jul 14;41(7):529-536. doi: 10.3760/cma.j.issn.0253-2727.2020.07.001. Zhonghua Xue Ye Xue Za Zhi. 2020. PMID: 32549120 Free PMC article. Chinese. No abstract available.
-
Low-dose antithymocyte globulin inhibits chronic graft-versus-host disease in peripheral blood stem cell transplantation from unrelated donors.Bone Marrow Transplant. 2021 Sep;56(9):2231-2240. doi: 10.1038/s41409-021-01314-w. Epub 2021 May 7. Bone Marrow Transplant. 2021. PMID: 33963304
-
Single- or double-unit UCBT following RIC in adults with AL: a report from Eurocord, the ALWP and the CTIWP of the EBMT.J Hematol Oncol. 2017 Jun 21;10(1):128. doi: 10.1186/s13045-017-0497-9. J Hematol Oncol. 2017. PMID: 28637512 Free PMC article.
-
Dynamic Graft-versus-Host Disease-Free, Relapse-Free Survival: Multistate Modeling of the Morbidity and Mortality of Allotransplantation.Biol Blood Marrow Transplant. 2019 Sep;25(9):1884-1889. doi: 10.1016/j.bbmt.2019.05.015. Epub 2019 May 22. Biol Blood Marrow Transplant. 2019. PMID: 31128328 Free PMC article. Clinical Trial.
-
Allogeneic peripheral blood stem cell transplantation with anti-thymocyte globulin versus allogeneic bone marrow transplantation without anti-thymocyte globulin.Haematologica. 2020 Apr;105(4):1138-1146. doi: 10.3324/haematol.2019.227603. Epub 2019 Aug 14. Haematologica. 2020. PMID: 31413093 Free PMC article.
References
-
- Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–61. doi: 10.1001/jama.2009.813. - DOI - PMC - PubMed
-
- Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9):3658–66. doi: 10.1182/blood-2006-06-025627. - DOI - PubMed
-
- de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857–64. doi: 10.1182/blood-2004-02-0414. - DOI - PubMed
-
- Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L, et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant. 2011;17(10):1490–6. doi: 10.1016/j.bbmt.2011.02.007. - DOI - PMC - PubMed
-
- Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14(6):672–84. doi: 10.1016/j.bbmt.2008.03.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

