In-vivo31P-MRS of skeletal muscle and liver: A way for non-invasive assessment of their metabolism
- PMID: 28119063
- PMCID: PMC5478074
- DOI: 10.1016/j.ab.2017.01.018
In-vivo31P-MRS of skeletal muscle and liver: A way for non-invasive assessment of their metabolism
Abstract
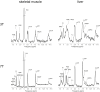
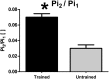
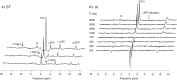
In addition to direct assessment of high energy phosphorus containing metabolite content within tissues, phosphorus magnetic resonance spectroscopy (31P-MRS) provides options to measure phospholipid metabolites and cellular pH, as well as the kinetics of chemical reactions of energy metabolism in vivo. Even though the great potential of 31P-MR was recognized over 30 years ago, modern MR systems, as well as new, dedicated hardware and measurement techniques provide further opportunities for research of human biochemistry. This paper presents a methodological overview of the 31P-MR techniques that can be used for basic, physiological, or clinical research of human skeletal muscle and liver in vivo. Practical issues of 31P-MRS experiments and examples of potential applications are also provided. As signal localization is essential for liver 31P-MRS and is important for dynamic muscle examinations as well, typical localization strategies for 31P-MR are also described.
Keywords: Energy metabolism; Exercise-recovery; Liver; Phosphorus magnetic resonance spectroscopy; Saturation transfer; Skeletal muscle.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ackerman J.J., Grove T.H., Wong G.G., Gadian D.G., Radda G.K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980;283:167–170. - PubMed
-
- Prompers J.J., Jeneson J.A., Drost M.R., Oomens C.C., Strijkers G.J., Nicolay K. Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR Biomed. 2006;19:927–953. - PubMed
-
- Brindle K.M., Blackledge M.J., Challiss R.A., Radda G.K. 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry. 1989;28:4887–4893. - PubMed
-
- Cadoux-Hudson T.A., Blackledge M.J., Radda G.K. Imaging of human brain creatine kinase activity in vivo. FASEB J. 1989;3:2660–2666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

