Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study
- PMID: 28119352
- PMCID: PMC5754855
- DOI: 10.1136/gutjnl-2016-312581
Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study
Abstract
Objective: The efficacy of anti-tumour necrosis factors (anti-TNFs) in patients with Crohn's disease (CD) and symptomatic small bowel stricture (SSBS) is controversial. The aim of this study was to estimate the efficacy of adalimumab in these patients and to identify the factors predicting success.
Design: We performed a multicentre, prospective, observational cohort study in patients with CD and SSBS. The included patients underwent magnetic resonance enterography at baseline and subsequently received adalimumab. The primary endpoint was success at week 24, defined as adalimumab continuation without prohibited treatment (corticosteroids after the eight week following inclusion, other anti-TNFs), endoscopic dilation or bowel resection. The baseline factors independently associated with success were identified using a logistic regression model, leading to a simple prognostic score. Secondary endpoints were prolonged success after week 24 (still on adalimumab, without dilation nor surgery) and time to bowel resection in the whole cohort.
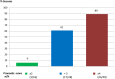
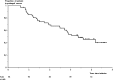
Results: From January 2010 to December 2011, 105 patients were screened and 97 were included. At week 24, 62/97 (64%) patients had achieved success. The prognostic score defined a good prognosis group with 43/49 successes, an intermediate prognosis group with 17/28 successes and a poor prognosis group with 1/16 successes. After a median follow-up time of 3.8 years, 45.7%±6.6% (proportion±SE) of patients who were in success at week 24 (ie, 29% of the whole cohort) were still in prolonged success at 4 years. Among the whole cohort, 50.7%±5.3% of patients did not undergo bowel resection 4 years after inclusion.
Conclusions: A successful response to adalimumab was observed in about two-thirds of CD patients with SSBS and was prolonged in nearly half of them till the end of follow-up. More than half of the patients were free of surgery 4 years after treatment initiation.
Clinical trial registration number: NCT01183403; Results.
Keywords: ABDOMINAL MRI; CROHN'S DISEASE; IBD CLINICAL.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: Consultancies: BMS, Shire, Sanofi, Norgine Pharma, MSD, Abbvie, Astra Zeneca, Roche, Takeda Millenium, Janssen Cilag, Pfizer Stock ownership: Inception IBD, San Diego, CA, USA Honoraria: BMS, MSD, Abbvie, Teva, Ferring, Vifor Pharma, HAC, Mayoli-Spindler Paid expert testimony: Abbvie Patent applications: None Travel grants: Abbvie, MSD, Ferring, Takeda, Vifor Pharma.
Figures
Comment in
-
Adalimumab in Crohn's strictures--the CREOLE study.Gut. 2018 Jan;67(1):198. doi: 10.1136/gutjnl-2017-314124. Epub 2017 Apr 5. Gut. 2018. PMID: 28381522 No abstract available.
-
Adalimumab in Crohn's disease and symptomatic small bowel strictures.Gut. 2018 Jan;67(1):199. doi: 10.1136/gutjnl-2017-314431. Epub 2017 Jun 10. Gut. 2018. PMID: 28601842 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
