Selective Ablation of Tumor Suppressors in Parafollicular C Cells Elicits Medullary Thyroid Carcinoma
- PMID: 28119454
- PMCID: PMC5339769
- DOI: 10.1074/jbc.M116.765727
Selective Ablation of Tumor Suppressors in Parafollicular C Cells Elicits Medullary Thyroid Carcinoma
Abstract
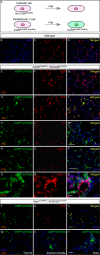
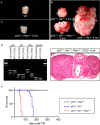
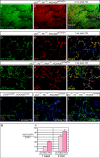
Among the four different types of thyroid cancer, treatment of medullary thyroid carcinoma poses a major challenge because of its propensity of early metastasis. To further investigate the molecular mechanisms of medullary thyroid carcinoma and discover candidates for targeted therapies, we developed a new mouse model of medullary thyroid carcinoma based on our CGRPCreER mouse line. This system enables gene manipulation in parafollicular C cells in the thyroid, the purported cells of origin of medullary thyroid carcinoma. Selective inactivation of tumor suppressors, such as p53, Rb, and Pten, in mature parafollicular C cells via an inducible Cre recombinase from CGRPCreER led to development of murine medullary thyroid carcinoma. Loss of Pten accelerated p53/Rb-induced medullary thyroid carcinoma, indicating interactions between pathways controlled by tumor suppressors. Moreover, labeling differentiated parafollicular C cells by CGRPCreER allows us to follow their fate during malignant transformation to medullary thyroid tumor. Our findings support a model in which mutational events in differentiated parafollicular C cells result in medullary thyroid carcinoma. Through expression analysis including RNA-Seq, we uncovered major signaling pathways and networks that are perturbed following the removal of tumor suppressors. Taken together, these studies not only increase our molecular understanding of medullary thyroid carcinoma but also offer new candidates for designing targeted therapies or other treatment modalities.
Keywords: CGRP; calcitonin; cancer biology; gene knock-out; genomics; medullary thyroid carcinoma; mouse genetics; parafollicular C cells; tumor suppressor gene.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Cabanillas M. E., McFadden D. G., and Durante C. (2016) Thyroid cancer. Lancet 388, 2783–2795 - PubMed
-
- Matias-Guiu X., and De Lellis R. (2014) Medullary thyroid carcinoma: a 25-year perspective. Endocr. Pathol. 25, 21–29 - PubMed
-
- Almeida M. Q., and Hoff A. O. (2012) Recent advances in the molecular pathogenesis and targeted therapies of medullary thyroid carcinoma. Curr. Opin. Oncol. 24, 229–234 - PubMed
-
- Wells S. A. Jr, Asa S. L., Dralle H., Elisei R., Evans D. B., Gagel R. F., Lee N., Machens A., Moley J. F., Pacini F., Raue F., Frank-Raue K., Robinson B., Rosenthal M. S., Santoro M., et al. (2015) Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25, 567–610 - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

