Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL
- PMID: 28119525
- PMCID: PMC5629361
- DOI: 10.1038/leu.2017.41
Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL
Abstract
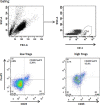
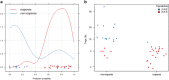
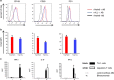
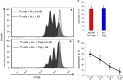
Blinatumomab can induce a complete haematological remission in patients in 46.6% with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL) resulting in a survival benefit when compared with chemotherapy. Only bone marrow blast counts before therapy have shown a weak prediction of response. Here we investigated the role of regulatory T cells (Tregs), measured by CD4/CD25/FOXP3 expression, in predicting the outcome of immunotherapy with the CD19-directed bispecific T-cell engager construct blinatumomab. Blinatumomab responders (n=22) had an average of 4.82% Tregs (confidence interval (CI): 1.79-8.34%) in the peripheral blood, whereas non-responders (n=20) demonstrated 10.25% Tregs (CI: 3.36-65.9%). All other tested markers showed either no prediction value or an inferior prediction level including blast BM counts and the classical enzyme marker lactate dehydrogenase. With a cutoff of 8.525%, Treg enumeration can identify 100% of all blinatumomab responders and exclude 70% of the non-responders. The effect is facilitated by blinatumomab-activated Tregs, leading to interleukin-10 production, resulting in suppression of T-cell proliferation and reduced CD8-mediated lysis of ALL cells. Proliferation of patients' T cells can be restored by upfront removal of Tregs. Thus, enumeration of Treg identifies r/r ALL patients with a high response rate to blinatumomab. Therapeutic removal of Tregs may convert blinatumomab non-responders to responders.
Conflict of interest statement
MST is an advisory board member, an honorary speaker and a travel support at Amgen. JD, TB, TM, MD, TD, HE, FE, FE, EH and AR declare no conflict of interest.
Figures


References
-
- Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, Durrant IJ et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109: 944–950. - PubMed
-
- Gökbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Hüttmann A et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120: 2032–2041. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

