Widespread Volumetric Brain Changes following Tooth Loss in Female Mice
- PMID: 28119577
- PMCID: PMC5220047
- DOI: 10.3389/fnana.2016.00121
Widespread Volumetric Brain Changes following Tooth Loss in Female Mice
Abstract
Tooth loss is associated with altered sensory, motor, cognitive and emotional functions. These changes vary highly in the population and are accompanied by structural and functional changes in brain regions mediating these functions. It is unclear to what extent this variability in behavior and function is caused by genetic and/or environmental determinants and which brain regions undergo structural plasticity that mediates these changes. Thus, the overall goal of our research program is to identify genetic variants that control structural and functional plasticity following tooth loss. As a step toward this goal, here our aim was to determine whether structural magnetic resonance imaging (sMRI) is sensitive to detect quantifiable volumetric differences in the brains of mice of different genetic background receiving tooth extraction or sham operation. We used 67 adult female mice of 7 strains, comprising the A/J (A) and C57BL/6J (B) strains and a randomly selected sample of 5 of the 23 AXB-BXA strains (AXB1, AXB4, AXB24, BXA14, BXA24) that were produced from the A and B parental mice by recombinations and inbreeding. This panel of 25 inbred strains of genetically diverse inbred strains of mice is used for mapping chromosomal intervals throughout the genome that harbor candidate genes controlling the phenotypic variance of any trait under study. Under general anesthesia, 39 mice received extraction of 3 right maxillary molar teeth and 28 mice received sham operation. On post-extraction day 21, post-mortem whole-brain high-resolution sMRI was used to quantify the volume of 160 brain regions. Compared to sham operation, tooth extraction was associated with a significantly reduced regional and voxel-wise volumes of cortical brain regions involved in processing somatosensory, motor, cognitive and emotional functions, and increased volumes in subcortical sensorimotor and temporal limbic forebrain regions including the amygdala. Additionally, comparison of the 10 BXA14 and 21 BXA24 mice revealed significant volumetric differences between the two strains in several brain regions. These findings highlight the utility of high-resolution sMRI for studying tooth loss-induced structural brain plasticity in mice, and provide a foundation for further phenotyping structural brain changes following tooth loss in the full AXB-BXA panel to facilitate mapping genes that control brain plasticity following orofacial injury.
Keywords: animal model; brain imaging (MRI); genetic variation; neuroplasticity; plasticity; sMRI; tooth loss; trigeminal nerve.
Figures
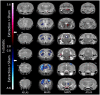
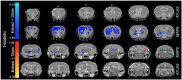
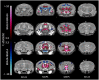
References
-
- Avivi-Arber L., Friedel M., Lerch J., Hayashi Y., Landzberg G., Moayedi M., et al. (2014). Loss of teeth in genetically-mapped recombinant inbred mouse strains as a model to study the genetic control of orofacial sensorimotor functions and the associated functional and sMRI-defined plasticity of the orofacial sensorimotor cortex post-injury, in Social Neuroscience Abstracts Annual Meeting (Washington, DC: ).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

