Trace Metals Derived from Electronic Cigarette (ECIG) Generated Aerosol: Potential Problem of ECIG Devices That Contain Nickel
- PMID: 28119618
- PMCID: PMC5222827
- DOI: 10.3389/fphys.2016.00663
Trace Metals Derived from Electronic Cigarette (ECIG) Generated Aerosol: Potential Problem of ECIG Devices That Contain Nickel
Abstract
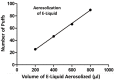
Introduction: ECIGs are currently under scrutiny concerning their safety, particularly in reference to the impact ECIG liquids (E-liquids) have on human health. One concern is that aerosolized E-liquids contain trace metals that could become trapped in respiratory tissues and induce pathology. Methods: To mimic this trapping, peristaltic pumps were used to generate and transport aerosol onto mixed cellulose ester (MCE) membranes where aluminum (Al), arsenic (As), cadmium (Cd), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), lead (Pb), and zinc (Zn) were subsequently captured and quantified. The presence of trace metals on unexposed MCE membranes and on MCE membranes exposed to mainstream smoke served as control and comparison, respectively. The presence of these metals was also determined from the E-liquid before aerosolization and untouched by the ECIG device. All metals were quantified using ICP-MS. The ECIG core assembly was analyzed using scanning electron microscopy with elemental analysis capability. Results: The contents (μg) of Al, As, Cd, Cu, Fe, Mn, Ni, Pb, and Zn on control MCE membranes were 1.2 ± 0.2, 0.050 ± 0.002, 0.047 ± 0.003, 0.05 ± 0.01, 0.001 ± 0.001, 0.16 ± 0.04, 0.005 ± 0.003, 0.014 ± 0.006, and 0.09 ± 0.02, respectively. The contents of all trace metals on MCE membranes exposed to aerosol were similar to controls, except Ni which was significantly (p < 0.01) higher (0.024 ± 0.004 μg). In contrast, contents of Al, As, Fe, Mn, and Zn on MCE membranes exposed to smoke were significantly higher (p < 0.05) than controls. The contents of Al, As, Cu, Fe, and Mn on smoke-exposed MCE membranes were also significantly higher (p < 0.05) than their content on aerosol-exposed membranes. The contents per cigarette equivalent of metals in E-liquid before aerosolization were negligible compared to amounts of aerosolized E-liquid, except for Fe (0.002 μg before and 0.001 μg after). Elemental analysis of the core assembly reveals the presence of several of these trace metals, especially Al, Fe, Ni, and Zn. Conclusions: In general, from the single ECIG-device/E-liquid combination used, the amount of trace metals from ECIG-generated aerosol are lower than in traditional mainstream smoke, Only Ni in the ECIG-generated aerosol was higher than control. The most probable source of Ni in this aerosol is the core assembly.
Keywords: E-liquid; ECIG; aerosol; smoking; trace metals; vaping.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

