Physiological Responses to Two Hypoxic Conditioning Strategies in Healthy Subjects
- PMID: 28119623
- PMCID: PMC5222853
- DOI: 10.3389/fphys.2016.00675
Physiological Responses to Two Hypoxic Conditioning Strategies in Healthy Subjects
Abstract
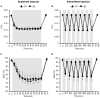
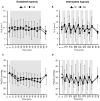
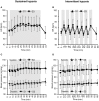
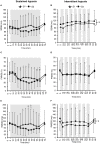
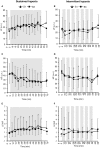
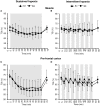
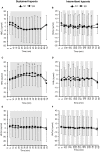
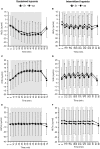
Objective: Hypoxic exposure can be used as a therapeutic tool by inducing various cardiovascular, neuromuscular, and metabolic adaptations. Hypoxic conditioning strategies have been evaluated in patients with chronic diseases using either sustained (SH) or intermittent (IH) hypoxic sessions. Whether hypoxic conditioning via SH or IH may induce different physiological responses remains to be elucidated. Methods: Fourteen healthy active subjects (7 females, age 25 ± 8 years, body mass index 21.5 ± 2.5 kg·m-2) performed two interventions in a single blind, randomized cross-over design, starting with either 3 x SH (48 h apart), or 3 x IH (48 h apart), separated by a 2 week washout period. SH sessions consisted of breathing a gas mixture with reduced inspiratory oxygen fraction (FiO2), continuously adjusted to reach arterial oxygen saturations (SpO2) of 70-80% for 1 h. IH sessions consisted of 5 min with reduced FiO2 (SpO2 = 70-80%), followed by 3-min normoxia, repeated seven times. During the first (S1) and third (S3) sessions of each hypoxic intervention, cardiorespiratory parameters, and muscle and pre-frontal cortex oxygenation (near infrared spectroscopy) were assessed continuously. Results: Minute ventilation increased significantly during IH sessions (+2 ± 2 L·min-1) while heart rate increased during both SH (+11 ± 4 bpm) and IH (+13 ± 5 bpm) sessions. Arterial blood pressure increased during all hypoxic sessions, although baseline normoxic systolic blood pressure was reduced from S1 to S3 in IH only (-8 ± 11 mmHg). Muscle oxygenation decreased significantly during S3 but not S1, for both hypoxic interventions (S3: SH -6 ± 5%, IH -3 ± 4%); pre-frontal oxygenation decreased in S1 and S3, and to a greater extent in SH vs. IH (-13 ± 3% vs. -6 ± 6%). Heart rate variability indices indicated a significantly larger increase in sympathetic activity in SH vs. IH (lower SDNN, PNN50, and RMSSD values in SH). From S1 to S3, further reduction in heart rate variability was observed in SH (SDNN, PNN50, and RMSSD reduction) while heart rate variability increased in IH (SDNN and RMSSD increase). Conclusions: These results showed significant differences in heart rate variability, blood pressure, and tissue oxygenation changes during short-term SH vs. IH conditioning interventions. Heart rate variability may provide useful information about the early adaptations induced by such intervention.
Keywords: blood pressure; heart rate variability; hypoxic conditioning; intermittent hypoxia; sustained hypoxia; tissue oxygenation.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

