Absence of tmRNA Has a Protective Effect against Fluoroquinolones in Streptococcus pneumoniae
- PMID: 28119681
- PMCID: PMC5222879
- DOI: 10.3389/fmicb.2016.02164
Absence of tmRNA Has a Protective Effect against Fluoroquinolones in Streptococcus pneumoniae
Abstract
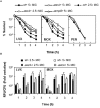
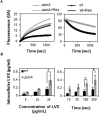
The transfer messenger RNA (tmRNA), encoded by the ssrA gene, is a small non-coding RNA involved in trans-translation that contributes to the recycling of ribosomes stalled on aberrant mRNAs. In most bacteria, its inactivation has been related to a decreased ability to respond to and recover from a variety of stress conditions. In this report, we investigated the role of tmRNA in stress adaptation in the human pathogen Streptococcus pneumoniae. We constructed a tmRNA deletion mutant and analyzed its response to several lethal stresses. The ΔssrA strain grew slower than the wild type, indicating that, although not essential, tmRNA is important for normal pneumococcal growth. Moreover, deletion of tmRNA increased susceptibility to UV irradiation, to exogenous hydrogen peroxide and to antibiotics that inhibit protein synthesis and transcription. However, the ΔssrA strain was more resistant to fluoroquinolones, showing twofold higher MIC values and up to 1000-fold higher survival rates than the wild type. Deletion of SmpB, the other partner in trans-translation, also reduced survival to levofloxacin in a similar extent. Accumulation of intracellular reactive oxygen species associated to moxifloxacin and levofloxacin treatment was also highly reduced (∼100-fold). Nevertheless, the ΔssrA strain showed higher intracellular accumulation of ethidium bromide and levofloxacin than the wild type, suggesting that tmRNA deficiency protects pneumococcal cells from fluoroquinolone-mediated killing. In fact, analysis of chromosome integrity revealed that deletion of tmRNA prevented the fragmentation of the chromosome associated to levofloxacin treatment. Moreover, such protective effect appears to relay mainly on inhibition of protein synthesis, since a similar effect was observed with antibiotics that inhibit that process. The emergence and spread of drug-resistant pneumococci is a matter of concern and these results contribute to a better comprehension of the mechanisms underlying fluoroquinolones action.
Keywords: Streptococcus pneumoniae; antibiotic resistance; chromosomal fragmentation; fluoroquinolones; reactive oxygen species; stress adaptation; tmRNA; trans-translation.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

