Quantitative iTRAQ Proteomics Revealed Possible Roles for Antioxidant Proteins in Sorghum Aluminum Tolerance
- PMID: 28119720
- PMCID: PMC5220100
- DOI: 10.3389/fpls.2016.02043
Quantitative iTRAQ Proteomics Revealed Possible Roles for Antioxidant Proteins in Sorghum Aluminum Tolerance
Abstract
Aluminum (Al) toxicity inhibits root growth and limits crop yields on acid soils worldwide. However, quantitative information is scarce on protein expression profiles under Al stress in crops. In this study, we report on the identification of potential Al responsive proteins from root tips of Al sensitive BR007 and Al tolerant SC566 sorghum lines using a strategy employing iTRAQ and 2D-liquid chromatography (LC) coupled to MS/MS (2D-LC-MS/MS). A total of 771 and 329 unique proteins with abundance changes of >1.5 or <0.67-fold were identified in BR007 and SC566, respectively. Protein interaction and pathway analyses indicated that proteins involved in the antioxidant system were more abundant in the tolerant line than in the sensitive one after Al treatment, while opposite trends were observed for proteins involved in lignin biosynthesis. Higher levels of ROS accumulation in root tips of the sensitive line due to decreased activity of antioxidant enzymes could lead to higher lignin production and hyper-accumulation of toxic Al in cell walls. These results indicated that activities of peroxidases and the balance between production and consumption of ROS could be important for Al tolerance and lignin biosynthesis in sorghum.
Keywords: aluminum tolerance; antioxidant system; lignin biosynthesis; proteomics; sorghum.
Figures
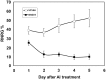

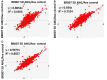

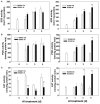
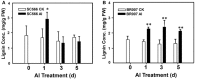

References
-
- Alban A., David S. O., Bjorkesten L., Andersson C., Sloge E., Lewis S., et al. . (2003). A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics 3, 36–44. 10.1002/pmic.200390006 - DOI - PubMed
-
- Castagliola P. (1998). Approximation of the normal sample median distribution using symmetrical Johnson SU distributions: application to quality control. Commun. Stat. Simulat. Comput. 27, 289–301. 10.1080/03610919808813481 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

