Euphorbia factor L2 induces apoptosis in A549 cells through the mitochondrial pathway
- PMID: 28119809
- PMCID: PMC5237708
- DOI: 10.1016/j.apsb.2016.06.008
Euphorbia factor L2 induces apoptosis in A549 cells through the mitochondrial pathway
Abstract
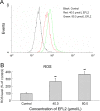
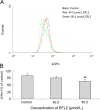
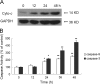
Euphorbia factor L2, a lathyrane diterpenoid isolated from caper euphorbia seed (the seeds of Euphorbia lathyris L.), has been traditionally applied to treat cancer. This article focuses on the cytotoxic activity of Euphorbia factor L2 against lung carcinoma A549 cells and the mechanism by which apoptosis is induced. We analyzed the cytotoxicity and related mechanism of Euphorbia factor L2 with an MTT assay, an annexin V-FITC/PI test, a colorimetric assay, and immunoblotting. Euphorbia factor L2 showed potent cytotoxicity to A549 cells. Euphorbia factor L2 led to an increase in reactive oxygen species (ROS) generation, a loss of mitochondrial electrochemical potential, release of cytochrome c, activation of caspase-9 and caspase-3, and cleavage of poly(ADP-ribose) polymerase, suggesting that Euphorbia factor L2 induced apoptosis through a mitochondrial pathway. The cytotoxic activity of Euphorbia factor L2 in A549 cells and the related mechanisms of apoptotic induction provide support for the further investigation of caper euphorbia seeds.
Keywords: Anticancer agent; Apoptosis; Caper euphorbia seed; Euphorbia Factor L2; Euphorbia lathyris L.; Mitochondrial pathway.
Figures



References
-
- Li L., Leung P.S. Use of herbal medicines and natural products: an alternative approach to overcoming the apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol. 2014;53:224–236. - PubMed
-
- Hong S.H., Ismail I.A., Kang S.M., Han D.C., Kwon B.M. Cinnamaldehydes in cancer chemotherapy. Phytother Res. 2016;30:754–767. - PubMed
-
- Ernst M., Grace O.M., Saslis-Lagoudakis C.H., Nilsson N., Simonsen H.T., Rønsted N. Global medicinal uses of Euphorbia L. (Euphorbiaceae) J Ethnopharmacol. 2015;176:90–101. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

