Urinary haptoglobin, alpha-1 anti-chymotrypsin and retinol binding protein identified by proteomics as potential biomarkers for lupus nephritis
- PMID: 28120479
- PMCID: PMC5383437
- DOI: 10.1111/cei.12930
Urinary haptoglobin, alpha-1 anti-chymotrypsin and retinol binding protein identified by proteomics as potential biomarkers for lupus nephritis
Abstract
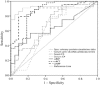
The study was aimed at identification by proteomics and validation by enzyme-linked immunosorbent assay (ELISA) of potential urinary biomarkers for lupus nephritis. Study subjects comprised 88 systemic lupus erythematosus (SLE) patients and 60 controls (rheumatoid arthritis, diabetes mellitus and healthy individuals). Based on the SLE disease activity index (SLEDAI), patients were classified as active renal (AR), active non-renal (ANR) or inactive disease (ID). Urinary proteins from a group of patients with AR or ID were resolved by two-dimensional gel electrophoresis and identified by matrix-assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS/MS). The selected biomarkers were validated by ELISA using samples from all patients and controls. AR patients were followed-up for 12 months after start of therapy. Three urinary proteins, alpha-1 anti-chymotrypsin (ACT), haptoglobin (HAP) and retinol binding protein (RBP), were detected in patients with AR and not ID. Upon validation, ACT levels were higher in AR patients than the other groups (P < 0·001) and showed good correlation with renal SLEDAI (r = 0·577, P < 0·001) as well as SLEDAI (r = 0·461, P < 0·001). Similarly, HAP levels were > 10-fold higher in AR than other groups (P < 0·001) and correlated well with renal SLEDAI (r = 0·594, P < 0·001) and SLEDAI (r = 0·371, P < 0·01). RBP levels were also higher in AR patients than in other groups (P < 0·05), except diabetes, and showed moderate correlation with renal SLEDAI (r = 0·284, P < 0·008) and SLEDAI (r = 0·316, P < 0·003). Upon follow-up with treatment, levels of all three proteins declined at 6 and 12 months (P < 0·01). Multiple logistic regression identified ACT as the best marker to differentiate AR from ANR. Urinary HAP, ACT and RBP are potential biomarkers for lupus nephritis activity.
Keywords: biomarkers; cyclophosphamide; glomerulonephritis; systemic lupus erythematosus.
© 2017 British Society for Immunology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

