Sizing clinical trials when comparing bivariate time-to-event outcomes
- PMID: 28120524
- PMCID: PMC5533151
- DOI: 10.1002/sim.7225
Sizing clinical trials when comparing bivariate time-to-event outcomes
Abstract
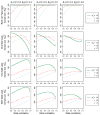
Clinical trials with multiple primary time-to-event outcomes are common. Use of multiple endpoints creates challenges in the evaluation of power and the calculation of sample size during trial design particularly for time-to-event outcomes. We present methods for calculating the power and sample size for randomized superiority clinical trials with two correlated time-to-event outcomes. We do this for independent and dependent censoring for three censoring scenarios: (i) the two events are non-fatal; (ii) one event is fatal (semi-competing risk); and (iii) both are fatal (competing risk). We derive the bivariate log-rank test in all three censoring scenarios and investigate the behavior of power and the required sample sizes. Separate evaluations are conducted for two inferential goals, evaluation of whether the test intervention is superior to the control on: (1) all of the endpoints (multiple co-primary) or (2) at least one endpoint (multiple primary). Copyright © 2017 John Wiley & Sons, Ltd.
Keywords: dependent censoring; log-rank test; multiple endpoints; semi-competing risk; time-dependent association.
Copyright © 2017 John Wiley & Sons, Ltd.
Figures
References
-
- Fine JP, Jiang H, Chappell R. On semi-competing risks data. Biometrika. 2001;88:907–919. doi: 10.1093/biomet/88.4.907. - DOI
-
- Offen W, Chuang-Stein C, Dmitrienko A, Littman G, Maca J, Meyerson L, Muirhead R, Stryszak P, Boddy A, Chen K, Copley-Merriman K, Dere W, Givens S, Hall D, Henry D, Jackson JD, Krishen A, Liu T, Ryder S, Sankoh AJ, Wang J, Yeh CH. Multiple co-primary endpoints: medical and statistical solutions. Drug Information Journal. 2007;41:31–46. doi: 10.1177/009286150704100105. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
