Phosphorylated Histone 3 at Serine 10 Identifies Activated Spinal Neurons and Contributes to the Development of Tissue Injury-Associated Pain
- PMID: 28120884
- PMCID: PMC5264160
- DOI: 10.1038/srep41221
Phosphorylated Histone 3 at Serine 10 Identifies Activated Spinal Neurons and Contributes to the Development of Tissue Injury-Associated Pain
Abstract
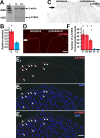
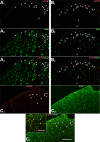
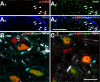
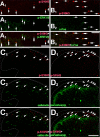
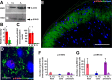
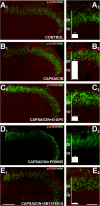
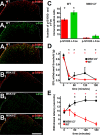
Transcriptional changes in superficial spinal dorsal horn neurons (SSDHN) are essential in the development and maintenance of prolonged pain. Epigenetic mechanisms including post-translational modifications in histones are pivotal in regulating transcription. Here, we report that phosphorylation of serine 10 (S10) in histone 3 (H3) specifically occurs in a group of rat SSDHN following the activation of nociceptive primary sensory neurons by burn injury, capsaicin application or sustained electrical activation of nociceptive primary sensory nerve fibres. In contrast, brief thermal or mechanical nociceptive stimuli, which fail to induce tissue injury or inflammation, do not produce the same effect. Blocking N-methyl-D-aspartate receptors or activation of extracellular signal-regulated kinases 1 and 2, or blocking or deleting the mitogen- and stress-activated kinases 1 and 2 (MSK1/2), which phosphorylate S10 in H3, inhibit up-regulation in phosphorylated S10 in H3 (p-S10H3) as well as fos transcription, a down-stream effect of p-S10H3. Deleting MSK1/2 also inhibits the development of carrageenan-induced inflammatory heat hyperalgesia in mice. We propose that p-S10H3 is a novel marker for nociceptive processing in SSDHN with high relevance to transcriptional changes and the development of prolonged pain.
Figures

References
-
- Breivik H., Collett B., Ventafridda V., Cohen R. & Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain 10, 287–333 (2006). - PubMed
-
- Ji R. R., Kohno T., Moore K. A. & Woolf C. J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705 (2003). - PubMed
-
- Ganguly K. & Poo M. M. Activity-dependent neural plasticity from bench to bedside. Neuron 80, 729–741 (2013). - PubMed
-
- Edelmayer R. M., Brederson J. D., Jarvis M. F. & Bitner R. S. Biochemical and pharmacological assessment of MAP-kinase signaling along pain pathways in experimental rodent models: a potential tool for the discovery of novel antinociceptive therapeutics. Biochem. Pharmacol. 87, 390–398 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

