Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection
- PMID: 28121710
- PMCID: PMC5373969
- DOI: 10.1097/QAD.0000000000001417
Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection
Abstract
Objective: To determine if immediate compared to deferred initiation of antiretroviral therapy (ART) in healthy persons living with HIV had a more favorable impact on health-related quality of life (QOL), or self-assessed physical, mental, and overall health status.
Design: QOL was measured in the Strategic Timing of Antiretroviral Therapy study, which randomized healthy ART-naive persons living with HIV with CD4 cell counts above 500 cells/μl from 35 countries to immediate versus deferred ART.
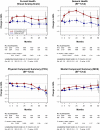
Methods: At baseline, months 4 and 12, then annually, participants completed a visual analog scale (VAS) for 'perceived current health' and the Short-Form 12-Item Health Survey version 2 from which the following were computed: general health perception; physical component summary (PCS); and mental component summary (MCS); the VAS and general health were rated from 0 (lowest) to 100 (highest).
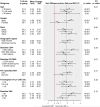
Results: QOL at study entry was high (mean scores: VAS = 80.9, general health = 72.5, PCS = 53.7, MCS = 48.2). Over a mean follow-up of 3 years, changes in all QOL measures favored the immediate group (P < 0.001); estimated differences were as follows: VAS = 1.9, general health = 3.6, PCS = 0.8, MCS = 0.9. When QOL changes were assessed across various demographic and clinical subgroups, treatment differences continued to favor the immediate group. QOL was poorer in those experiencing primary outcomes; however, when excluding those with primary events, results remained favorable for immediate ART recipients.
Conclusion: In an international randomized trial in ART-naive participants with above 500 CD4 cells/μl, there were modest but significant improvements in self-assessed QOL among those initiating ART immediately compared to deferring treatment, supporting patient-perceived health benefits of initiating ART as soon as possible after an HIV diagnosis.
Figures

References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed September 15, 2016.
-
- World Health Organization . Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. WHO; Geneva: 2015. www.who.int/hiv/pub/guidelines/earlyrelease-arv/en. Accessed September 15, 2016. - PubMed
-
- Preamble to the constitution of the World Health Organization as adopted by the International Health Conference; New York. 19 June – 22 July 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948.
-
- Cella D. Quality of life: Concepts and definition. J Pain Symptom Management. 1994;9(3):186–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

