The Iron-Dependent Regulation of the Candida albicans Oxidative Stress Response by the CCAAT-Binding Factor
- PMID: 28122000
- PMCID: PMC5266298
- DOI: 10.1371/journal.pone.0170649
The Iron-Dependent Regulation of the Candida albicans Oxidative Stress Response by the CCAAT-Binding Factor
Abstract
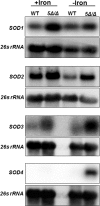
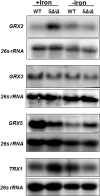
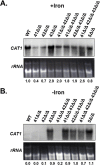
Candida albicans is the most frequently encountered fungal pathogen in humans, capable of causing mucocutaneous and systemic infections in immunocompromised individuals. C. albicans virulence is influenced by multiple factors. Importantly, iron acquisition and avoidance of the immune oxidative burst are two critical barriers for survival in the host. Prior studies using whole genome microarray expression data indicated that the CCAAT-binding factor is involved in the regulation of iron uptake/utilization and the oxidative stress response. This study examines directly the role of the CCAAT-binding factor in regulating the expression of oxidative stress genes in response to iron availability. The CCAAT-binding factor is a heterooligomeric transcription factor previously shown to regulate genes involved in respiration and iron uptake/utilization in C. albicans. Since these pathways directly influence the level of free radicals, it seemed plausible the CCAAT-binding factor regulates genes necessary for the oxidative stress response. In this study, we show the CCAAT-binding factor is involved in regulating some oxidative stress genes in response to iron availability, including CAT1, SOD4, GRX5, and TRX1. We also show that CAT1 expression and catalase activity correlate with the survival of C. albicans to oxidative stress, providing a connection between iron obtainability and the oxidative stress response. We further explore the role of the various CCAAT-binding factor subunits in the formation of distinct protein complexes that modulate the transcription of CAT1 in response to iron. We find that Hap31 and Hap32 can compensate for each other in the formation of an active transcriptional complex; however, they play distinct roles in the oxidative stress response during iron limitation. Moreover, Hap43 was found to be solely responsible for the repression observed under iron deprivation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Odds FC, editor. Candida and candidosis, 2nd Ed. London, United Kingdom: Bailliere Tindall; 1988.
-
- Calderone RA, editor. Candida and candidiasis. Washington, D.C.: ASM Press; 2002.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

