The Effect of Tacrolimus and Mycophenolic Acid on CD14+ Monocyte Activation and Function
- PMID: 28122021
- PMCID: PMC5266297
- DOI: 10.1371/journal.pone.0170806
The Effect of Tacrolimus and Mycophenolic Acid on CD14+ Monocyte Activation and Function
Abstract
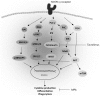
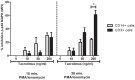
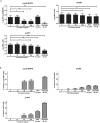
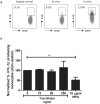
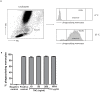
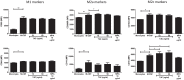
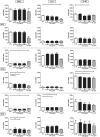
Monocytes and macrophages play key roles in many disease states, including cellular and humoral rejection after solid organ transplantation (SOT). To suppress alloimmunity after SOT, immunosuppressive drug therapy is necessary. However, little is known about the effects of the immunosuppressive drugs tacrolimus and mycophenolic acid (MPA) on monocyte activation and function. Here, the effect of these immunosuppressants on monocytes was investigated by measuring phosphorylation of three intracellular signaling proteins which all have a major role in monocyte function: p38MAPK, ERK and Akt. In addition, biological functions downstream of these signaling pathways were studied, including cytokine production, phagocytosis and differentiation into macrophages. To this end, blood samples from healthy volunteers were spiked with diverse concentrations of tacrolimus and MPA. Tacrolimus (200 ng/ml) inhibited phosphorylation of p38MAPK by 30% (mean) in CD14+ monocytes which was significantly less than in activated CD3+ T cells (max 60%; p < 0.05). This immunosuppressive agent also partly inhibited p-AKT (14%). MPA, at a therapeutic concentration showed the strongest effect on p-AKT (27% inhibition). p-ERK was inhibited with a maximum of 15% after spiking with either tacrolimus or MPA. The production of IL-1β and phagocytosis by monocytes were not affected by tacrolimus concentrations, whereas MPA did inhibit IL-1β production by 50%. Monocyte/macrophage polarization was shifted to an M2-like phenotype in the presence of tacrolimus, while MPA increased the expression of M2 surface markers, including CD163 and CD200R, on M1 macrophages. These results show that tacrolimus and MPA do not strongly affect monocyte function, apart from a change in macrophage polarization, to a clinically relevant degree.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: D.A. Hesselink has received lecture fees and grant support from Astellas Pharma. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Pharmacodynamic Monitoring of Tacrolimus-Based Immunosuppression in CD14+ Monocytes After Kidney Transplantation.Ther Drug Monit. 2017 Oct;39(5):463-471. doi: 10.1097/FTD.0000000000000426. Ther Drug Monit. 2017. PMID: 28640063
-
Mycophenolic acid differentially affects dendritic cell maturation induced by tumor necrosis factor-alpha and lipopolysaccharide through a different modulation of MAPK signaling.Mol Immunol. 2010 May;47(9):1848-59. doi: 10.1016/j.molimm.2009.10.026. Epub 2010 Mar 23. Mol Immunol. 2010. PMID: 20334925
-
Susceptibility of human mesenchymal stem cells to tacrolimus, mycophenolic acid, and rapamycin.Transplantation. 2008 Nov 15;86(9):1283-91. doi: 10.1097/TP.0b013e31818aa536. Transplantation. 2008. PMID: 19005411
-
Molecular mechanisms of new immunosuppressants.Clin Transplant. 1996 Feb;10(1 Pt 2):118-23. Clin Transplant. 1996. PMID: 8680047 Review.
-
Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways.Cell Signal. 2014 Feb;26(2):192-7. doi: 10.1016/j.cellsig.2013.11.004. Epub 2013 Nov 9. Cell Signal. 2014. PMID: 24219909 Review.
Cited by
-
Immunosuppressant imprecision: multidirectional effects on metabolism and microbiome.Clin Microbiol Rev. 2025 Jun 12;38(2):e0017824. doi: 10.1128/cmr.00178-24. Epub 2025 Mar 5. Clin Microbiol Rev. 2025. PMID: 40042298 Review.
-
Effects of Antirejection Drugs on Innate Immune Cells After Kidney Transplantation.Front Immunol. 2019 Dec 19;10:2978. doi: 10.3389/fimmu.2019.02978. eCollection 2019. Front Immunol. 2019. PMID: 31921213 Free PMC article. Review.
-
Analysis and Fine Specificity of the HCMV-Specific Cell-Free and Cell-Associated Antibody-Dependent Cellular Phagocytosis (ADCP) Responses in Lung Transplant Recipients.Int J Mol Sci. 2021 Jul 30;22(15):8206. doi: 10.3390/ijms22158206. Int J Mol Sci. 2021. PMID: 34360986 Free PMC article.
-
Role of donor macrophages after heart and lung transplantation.Am J Transplant. 2020 May;20(5):1225-1235. doi: 10.1111/ajt.15751. Epub 2020 Jan 29. Am J Transplant. 2020. PMID: 31850651 Free PMC article. Review.
-
Pimecrolimus interferes the therapeutic efficacy of human mesenchymal stem cells in atopic dermatitis by regulating NFAT-COX2 signaling.Stem Cell Res Ther. 2021 Aug 28;12(1):482. doi: 10.1186/s13287-021-02547-8. Stem Cell Res Ther. 2021. PMID: 34454603 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

