Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia
- PMID: 28122118
- PMCID: PMC5388568
- DOI: 10.1002/phar.1906
Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia
Abstract
Objective: To compare and contrast the efficacy and safety of patiromer and sodium zirconium cyclosilicate (ZS-9) in the treatment of hyperkalemia.
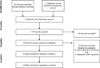
Design: A systematic review and meta-analysis of phase II and III clinical trial data was completed.
Patients or participants: Eight studies (two phase II and four phase III trials with two subgroup analyses) were included in the qualitative analysis, and six studies (two phase II and four phase III trials) were included in the meta-analysis.
Measurements and results: Significant heterogeneity was found in the meta-analysis with an I2 value ranging from 80.6-99.6%. A random-effects meta-analysis was applied for all end points. Each clinical trial stratified results by hyperkalemia severity and dosing; therefore, these were considered separate treatment groups in the meta-analysis. For patiromer, a significant -0.70 mEq/L (95% confidence interval [CI] -0.48 to -0.91 mEq/L) change was noted in potassium at 4 weeks. At day 3 of patiromer treatment, potassium change was -0.36 mEq/L (range of standard deviation 0.07-0.30). The primary end point for ZS-9-change in potassium at 48 hours-was -0.67 mEq/L (95% CI -0.45 to -0.89 mEq/L). By 1 hour after ZS-9 administration, change in potassium was -0.17 mEq/L (95% CI -0.05 to -0.30). Analysis of pooled adverse effects from these trials indicates that patiromer was associated with more gastrointestinal upset (7.6% constipation, 4.5% diarrhea) and electrolyte depletion (7.1% hypomagnesemia), whereas ZS-9 was associated with the adverse effects of urinary tract infections (1.1%) and edema (0.9%).
Conclusion: Patiromer and ZS-9 represent significant pharmacologic advancements in the treatment of hyperkalemia. Both agents exhibited statistically and clinically significant reductions in potassium for the primary end point of this meta-analysis. Given the adverse effect profile and the observed time-dependent effects, ZS-9 may play more of a role in treating acute hyperkalemia.
Keywords: ZS-9; hyperkalemia; patiromer; sodium polystyrene sulfonate; sodium zirconium cyclosilicate.
© 2017 Pharmacotherapy Publications, Inc.
Conflict of interest statement
Disclosures: The authors have no actual or potential conflicts of interest to declare.
Figures




Similar articles
-
Potassium binders for chronic hyperkalaemia in people with chronic kidney disease.Cochrane Database Syst Rev. 2020 Jun 26;6(6):CD013165. doi: 10.1002/14651858.CD013165.pub2. Cochrane Database Syst Rev. 2020. PMID: 32588430 Free PMC article.
-
Sodium zirconium cyclosilicate versus sodium polystyrene sulfonate for treatment of hyperkalemia in hemodialysis patients: a randomized clinical trial.BMC Nephrol. 2025 May 6;26(1):227. doi: 10.1186/s12882-025-04129-9. BMC Nephrol. 2025. PMID: 40329202 Free PMC article. Clinical Trial.
-
Efficacy and safety of patiromer for hyperkalemia: a randomized, placebo-controlled phase 3 study.Clin Exp Nephrol. 2025 Jul;29(7):899-911. doi: 10.1007/s10157-025-02637-4. Epub 2025 Feb 20. Clin Exp Nephrol. 2025. PMID: 39976633 Free PMC article. Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Risk of Serious Adverse Gastrointestinal Events with Potassium Binders in Hospitalized Patients: A National Study.J Gen Intern Med. 2025 Feb;40(3):518-524. doi: 10.1007/s11606-024-08979-1. Epub 2024 Aug 5. J Gen Intern Med. 2025. PMID: 39103605
Cited by
-
Nephrotic Syndrome Complications - New and Old. Part 1.Maedica (Bucur). 2022 Mar;17(1):153-168. doi: 10.26574/maedica.2022.17.1.153. Maedica (Bucur). 2022. PMID: 35733752 Free PMC article.
-
Pharmacology of new treatments for hyperkalaemia: patiromer and sodium zirconium cyclosilicate.Eur Heart J Suppl. 2019 Feb;21(Suppl A):A28-A33. doi: 10.1093/eurheartj/suy035. Epub 2019 Feb 26. Eur Heart J Suppl. 2019. PMID: 30837802 Free PMC article.
-
Hyperkalemia management: a multidisciplinary expert panel's perspective on the role of new potassium binders.Heart Fail Rev. 2025 Mar;30(2):271-286. doi: 10.1007/s10741-024-10461-3. Epub 2024 Nov 27. Heart Fail Rev. 2025. PMID: 39604607 Free PMC article. Review.
-
Use of Fludrocortisone for Hyperkalemia in Chronic Kidney Disease Not Yet on Dialysis.Electrolyte Blood Press. 2024 Jun;22(1):8-15. doi: 10.5049/EBP.2024.22.1.8. Epub 2024 Jun 27. Electrolyte Blood Press. 2024. PMID: 38957547 Free PMC article.
-
Evaluation of potential drug interactions with sodium zirconium cyclosilicate: a single-center, open-label, one sequence crossover study in healthy adults.Clin Kidney J. 2020 Dec 29;14(7):1808-1816. doi: 10.1093/ckj/sfaa222. eCollection 2021 Jul. Clin Kidney J. 2020. PMID: 34221388 Free PMC article.
References
-
- Emmett M, Hootkins RE, Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology. 1995;3:752–760. - PubMed
-
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;5:733–735. - PubMed
-
- Gruy-Kapral C, Emmett M, Santa Ana CA, Porter JL, Fordtran JS, Fine KD. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol. 1998;10:1924–1930. - PubMed
-
- Cheng ES, Stringer KM, Pegg SP. Colonic necrosis and perforation following oral sodium polystyrene sulfonate (Resonium A/Kayexalate in a burn patient. Burns. 2002;2:189–190. - PubMed
-
- Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;2:159–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

