The Relaxation Properties of Myofibrils Are Compromised by Amino Acids that Stabilize α-Tropomyosin
- PMID: 28122223
- PMCID: PMC5266141
- DOI: 10.1016/j.bpj.2016.12.013
The Relaxation Properties of Myofibrils Are Compromised by Amino Acids that Stabilize α-Tropomyosin
Abstract
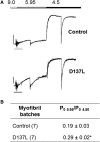
We investigated the functional impact of α-tropomyosin (Tm) substituted with one (D137L) or two (D137L/G126R) stabilizing amino acid substitutions on the mechanical behavior of rabbit psoas skeletal myofibrils by replacing endogenous Tm and troponin (Tn) with recombinant Tm mutants and purified skeletal Tn. Force recordings from myofibrils (15°C) at saturating [Ca2+] showed that Tm-stabilizing substitutions did not significantly affect the maximal isometric tension and the rates of force activation (kACT) and redevelopment (kTR). However, a clear effect was observed on force relaxation: myofibrils with D137L/G126R or D137L Tm showed prolonged durations of the slow phase of relaxation and decreased rates of the fast phase. Both Tm-stabilizing substitutions strongly decreased the slack sarcomere length (SL) at submaximal activating [Ca2+] and increased the steepness of the SL-passive tension relation. These effects were reversed by addition of 10 mM 2,3-butanedione 2-monoxime. Myofibrils also showed an apparent increase in Ca2+ sensitivity. Measurements of myofibrillar ATPase activity in the absence of Ca2+ showed a significant increase in the presence of these Tms, indicating that single and double stabilizing substitutions compromise the full inhibition of contraction in the relaxed state. These data can be understood with the three-state (blocked-closed-open) theory of muscle regulation, according to which the mutations increase the contribution of the active open state in the absence of Ca2+ (M-). Force measurements on myofibrils substituted with C-terminal truncated TnI showed similar compromised relaxation effects, indicating the importance of TnI-Tm interactions in maintaining the blocked state. It appears that reducing the flexibility of native Tm coiled-coil structure decreases the optimum interactions of the central part of Tm with the C-terminal region of TnI. This results in a shift away from the blocked state, allowing myosin binding and activity in the absence of Ca2+. This work provides a basis for understanding the effects of disease-producing mutations in muscle proteins.
Copyright © 2017. Published by Elsevier Inc.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

