Search strategy is regulated by somatostatin signaling and deep brain photoreceptors in zebrafish
- PMID: 28122559
- PMCID: PMC5267475
- DOI: 10.1186/s12915-016-0346-2
Search strategy is regulated by somatostatin signaling and deep brain photoreceptors in zebrafish
Abstract
Background: Animals use sensory cues to efficiently locate resources, but when sensory information is insufficient, they may rely on internally coded search strategies. Despite the importance of search behavior, there is limited understanding of the underlying neural mechanisms in vertebrates.
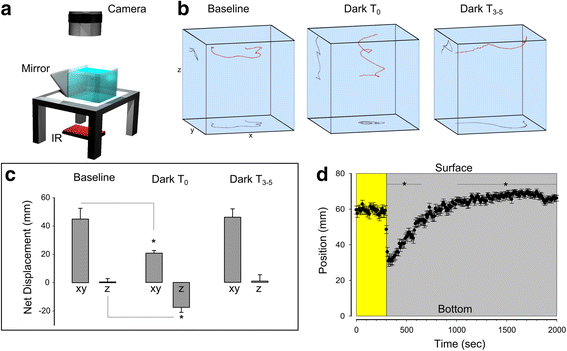
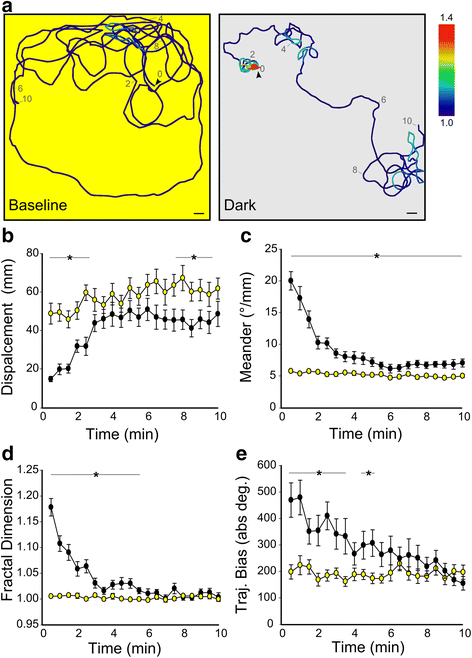
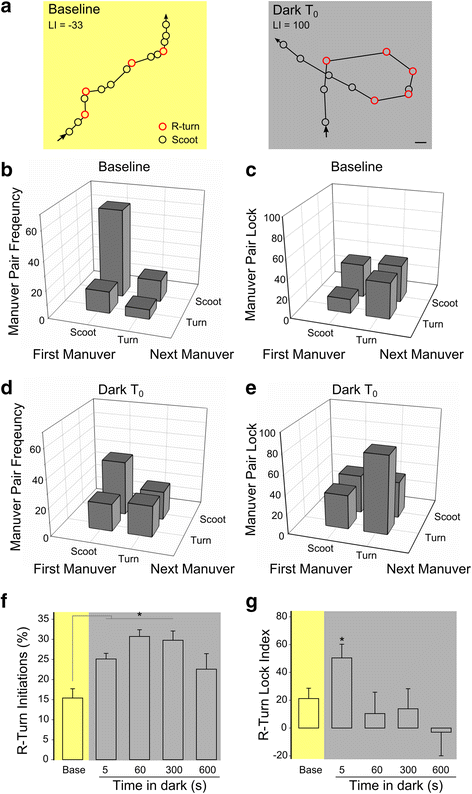
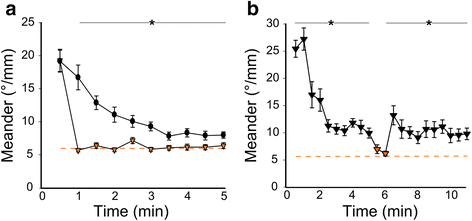
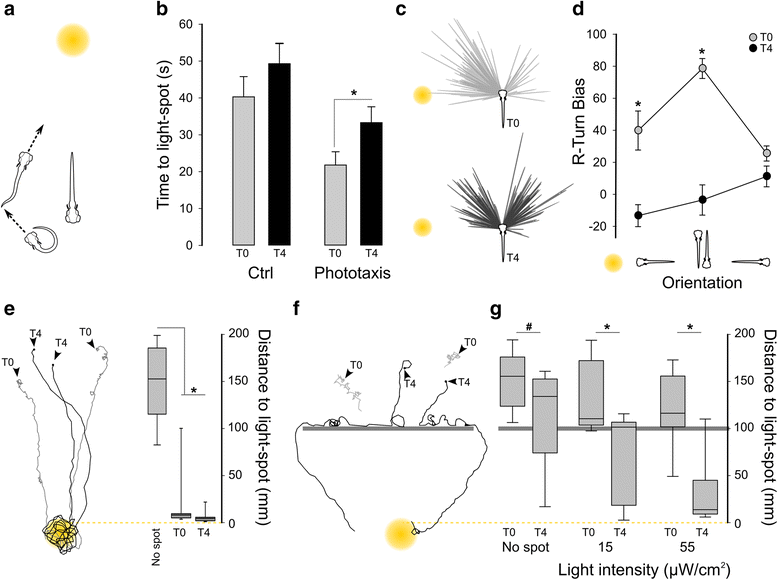
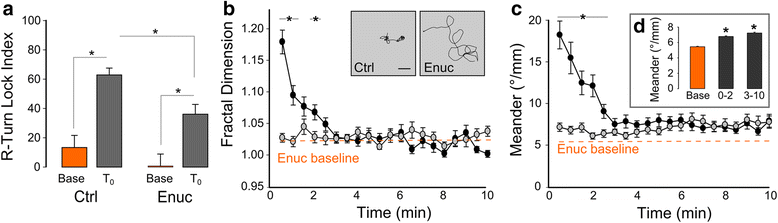
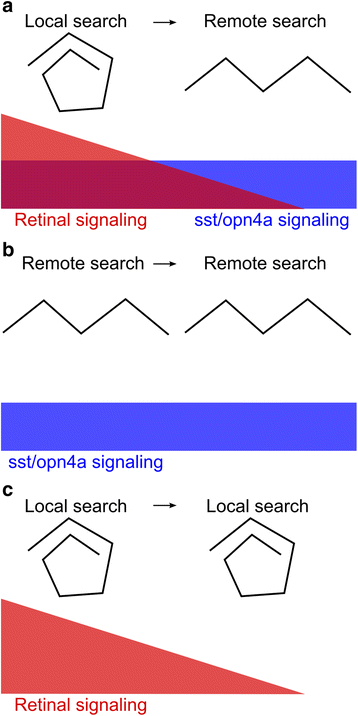
Results: Here, we report that loss of illumination initiates sophisticated light-search behavior in larval zebrafish. Using three-dimensional tracking, we show that at the onset of darkness larvae swim in a helical trajectory that is spatially restricted in the horizontal plane, before gradually transitioning to an outward movement profile. Local and outward swim patterns display characteristic features of area-restricted and roaming search strategies, differentially enhancing phototaxis to nearby and remote sources of light. Retinal signaling is only required to initiate area-restricted search, implying that photoreceptors within the brain drive the transition to the roaming search state. Supporting this, orthopediaA mutant larvae manifest impaired transition to roaming search, a phenotype which is recapitulated by loss of the non-visual opsin opn4a and somatostatin signaling.
Conclusion: These findings define distinct neuronal pathways for area-restricted and roaming search behaviors and clarify how internal drives promote goal-directed activity.
Keywords: CRISPR; Goal-directed behavior; Melanopsin; Motivation; Non-visual photoreceptor; Orthopedia; Search; Somatostatin; Zebrafish; opn4a; sst1.1.
Figures

References
-
- Bell WJ. Searching behaviour: The behavioural ecology of finding resources. London: Chapman and Hall; 1990.
-
- Bell WJ. Sources of information controllling motor patterns in arthropod local search orientation. J Insect Physiol. 1985;31(11):837–47. doi: 10.1016/0022-1910(85)90101-5. - DOI
-
- Wehner R, Srinivasan MV. Searching behavior of desert ants, genus cataglyphis (formicidae, hymenoptera) J Comp Physiol. 1981;142:315–38. doi: 10.1007/BF00605445. - DOI
-
- White J, Tobin TR, Bell WJ. Local search in the housefly musca domestica after feeding on sucrose. J Insect Physiol. 1984;30(6):477–87. doi: 10.1016/0022-1910(84)90028-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

