Pigment Epithelium-Derived Factor (PEDF) mediates cartilage matrix loss in an age-dependent manner under inflammatory conditions
- PMID: 28122611
- PMCID: PMC5264335
- DOI: 10.1186/s12891-017-1410-y
Pigment Epithelium-Derived Factor (PEDF) mediates cartilage matrix loss in an age-dependent manner under inflammatory conditions
Abstract
Background: Inflammation is a major cause of cartilage destruction and leads to the imbalance of metabolic activities in the arthritic joint. Pigment epithelium-derived factor (PEDF) has been reported to have both pro- and anti-inflammatory activities in various cell types and to be upregulated in the arthritic joint, but its role in joint destruction is unclear. Our aim was to investigate the role of PEDF in cartilage degeneration under inflammatory conditions.
Methods: PEDF was ectopically expressed in primary human articular chondrocytes, and catabolic gene expression and protein secretion in response to the pro-inflammatory cytokine interleukin 1 beta (IL-1β) were evaluated. Metatarsal bones from PEDF-deficient and wild type mice were cultured in the presence or absence of IL-1β. Cartilage matrix integrity and matrix metalloproteinases MMP-1, MMP-3, and MMP-13 were evaluated. PEDF-deficient and wild type mice were evaluated in the monosodium iodoacetate (MIA) inflammatory joint destruction animal model to determine the role of PEDF in inflammatory arthritis in vivo. Student's t-tests and Mann-Whitney tests were employed where appropriate, for parametric and non-parametric data, respectively.
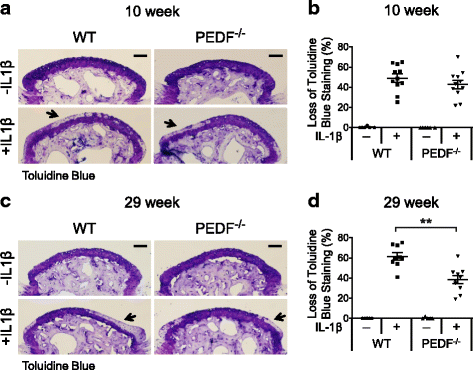
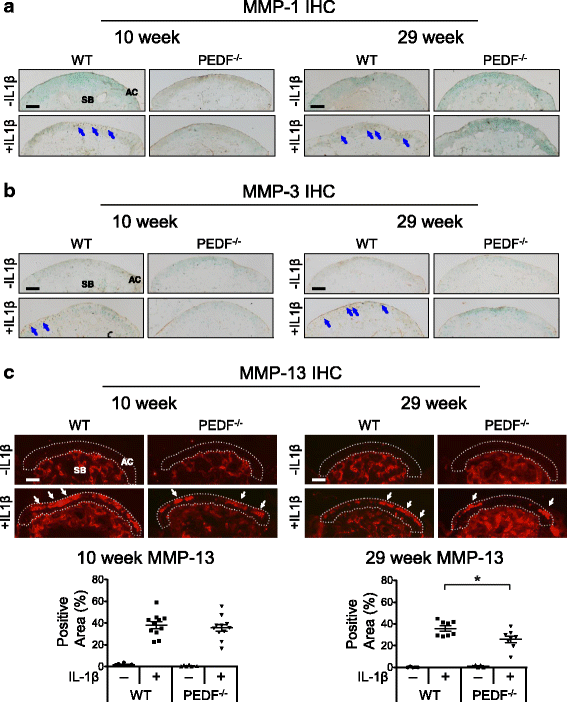
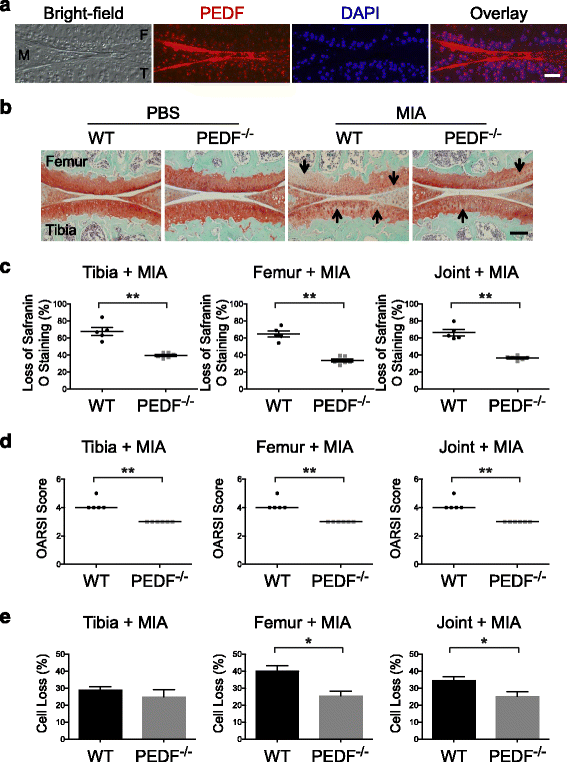
Results: We showed that PEDF protein levels were higher in human osteoarthritis samples compared to normal samples. We demonstrated that ectopic PEDF expression in primary human articular chondrocytes exacerbated catabolic gene expression in the presence of IL-1β. In whole bone organ cultures, IL-1β induced MMP-1, MMP-3 and MMP-13 protein production, and caused significant cartilage matrix loss. Interestingly, Toluidine Blue staining showed that PEDF-deficient bones from 29 week old animals, but not 10 week old animals, had reduced matrix loss in response to IL-1β compared to their wild type counterparts. In addition, PEDF-deficiency in 29 week old animals preserved matrix integrity and protected against cell loss in the MIA joint destruction model in vivo.
Conclusion: We conclude that PEDF exacerbates cartilage degeneration in an age-dependent manner under an inflammatory setting. This is the first study identifying a specific role for PEDF in joint inflammation and highlights the multi-faceted activities of PEDF.
Keywords: Age-related; Arthritis; IL-1β; Inflammation; MMPs; Matrix, cartilage; Pigment epithelium-derived factor; SERPINF1.
Figures



References
-
- Wong R, et al. Prevalence of Arthritis and Rheumatic Diseases Around the World: A Growing Burden and Implications for Health Care Needs. Models of Care in Arthritis (MOCA). 2010. Working Paper 10-02. http://modelsofcare.ca/resources.html#workingpaper.
-
- Joosten LA, et al. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163(9):5049–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

