Dynamic Consent: a potential solution to some of the challenges of modern biomedical research
- PMID: 28122615
- PMCID: PMC5264333
- DOI: 10.1186/s12910-016-0162-9
Dynamic Consent: a potential solution to some of the challenges of modern biomedical research
Abstract
Background: Innovations in technology have contributed to rapid changes in the way that modern biomedical research is carried out. Researchers are increasingly required to endorse adaptive and flexible approaches to accommodate these innovations and comply with ethical, legal and regulatory requirements. This paper explores how Dynamic Consent may provide solutions to address challenges encountered when researchers invite individuals to participate in research and follow them up over time in a continuously changing environment.
Methods: An interdisciplinary workshop jointly organised by the University of Oxford and the COST Action CHIP ME gathered clinicians, researchers, ethicists, lawyers, research participants and patient representatives to discuss experiences of using Dynamic Consent, and how such use may facilitate the conduct of specific research tasks. The data collected during the workshop were analysed using a content analysis approach.
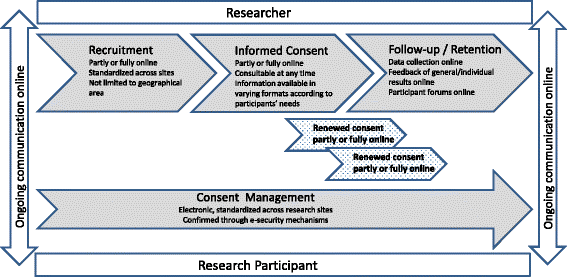
Results: Dynamic Consent can provide practical, sustainable and future-proof solutions to challenges related to participant recruitment, the attainment of informed consent, participant retention and consent management, and may bring economic efficiencies.
Conclusions: Dynamic Consent offers opportunities for ongoing communication between researchers and research participants that can positively impact research. Dynamic Consent supports inter-sector, cross-border approaches and large scale data-sharing. Whilst it is relatively easy to set up and maintain, its implementation will require that researchers re-consider their relationship with research participants and adopt new procedures.
Keywords: Biobank; Clinical research; Clinical trials; Dynamic consent; Ethics; Participant engagement; Research communication; Software tools.
Figures
References
-
- European Science Foundation. ESF Forward Look: Personalised Medicine for the European Citizen. http://archives.esf.org/fileadmin/Public_documents/Publications/Personal.... Accessed 25 Nov 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources