Primary cilia regulate the osmotic stress response of renal epithelial cells through TRPM3
- PMID: 28122715
- PMCID: PMC5407065
- DOI: 10.1152/ajprenal.00465.2015
Primary cilia regulate the osmotic stress response of renal epithelial cells through TRPM3
Abstract
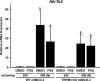
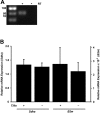
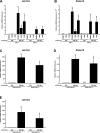
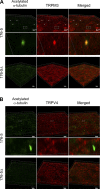
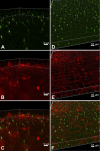
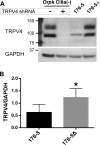
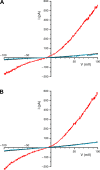
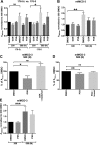
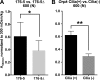
Primary cilia sense environmental conditions, including osmolality, but whether cilia participate in the osmotic response in renal epithelial cells is not known. The transient receptor potential (TRP) channels TRPV4 and TRPM3 are osmoresponsive. TRPV4 localizes to cilia in certain cell types, while renal subcellular localization of TRPM3 is not known. We hypothesized that primary cilia are required for maximal activation of the osmotic response of renal epithelial cells and that ciliary TRPM3 and TRPV4 mediate that response. Ciliated [murine epithelial cells from the renal inner medullary collecting duct (mIMCD-3) and 176-5] and nonciliated (176-5Δ) renal cells expressed Trpv4 and Trpm3 Ciliary expression of TRPM3 was observed in mIMCD-3 and 176-5 cells and in wild-type mouse kidney tissue. TRPV4 was identified in cilia and apical membrane of mIMCD-3 cells by electrophysiology and in the cell body by immunofluorescence. Hyperosmolal stress at 500 mOsm/kg (via NaCl addition) induced the osmotic response genes betaine/GABA transporter (Bgt1) and aldose reductase (Akr1b3) in all ciliated cell lines. This induction was attenuated in nonciliated cells. A TRPV4 agonist abrogated Bgt1 and Akr1b3 induction in ciliated and nonciliated cells. A TRPM3 agonist attenuated Bgt1 and Akr1b3 induction in ciliated cells only. TRPM3 knockout attenuated Akr1b3 induction. Viability under osmotic stress was greater in ciliated than nonciliated cells. Akr1b3 induction was also less in nonciliated than ciliated cells when mannitol was used to induce hyperosmolal stress. These findings suggest that primary cilia are required for the maximal osmotic response in renal epithelial cells and that TRPM3 is involved in this mechanism. TRPV4 appears to modulate the osmotic response independent of cilia.
Keywords: TRPM3; TRPV4; osmoregulation; primary cilium.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Bagnasco S, Balaban R, Fales HM, Yang YM, Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem 261: 5872–5877, 1986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

