Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia
- PMID: 28122742
- PMCID: PMC5356455
- DOI: 10.1182/blood-2016-09-738070
Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia
Abstract
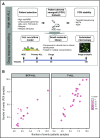
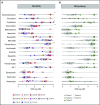
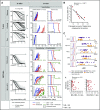
Drug sensitivity and resistance testing on diagnostic leukemia samples should provide important functional information to guide actionable target and biomarker discovery. We provide proof of concept data by profiling 60 drugs on 68 acute lymphoblastic leukemia (ALL) samples mostly from resistant disease in cocultures of bone marrow stromal cells. Patient-derived xenografts retained the original pattern of mutations found in the matched patient material. Stromal coculture did not prevent leukemia cell cycle activity, but a specific sensitivity profile to cell cycle-related drugs identified samples with higher cell proliferation both in vitro and in vivo as leukemia xenografts. In patients with refractory relapses, individual patterns of marked drug resistance and exceptional responses to new agents of immediate clinical relevance were detected. The BCL2-inhibitor venetoclax was highly active below 10 nM in B-cell precursor ALL (BCP-ALL) subsets, including MLL-AF4 and TCF3-HLF ALL, and in some T-cell ALLs (T-ALLs), predicting in vivo activity as a single agent and in combination with dexamethasone and vincristine. Unexpected sensitivity to dasatinib with half maximal inhibitory concentration values below 20 nM was detected in 2 independent T-ALL cohorts, which correlated with similar cytotoxic activity of the SRC inhibitor KX2-391 and inhibition of SRC phosphorylation. A patient with refractory T-ALL was treated with dasatinib on the basis of drug profiling information and achieved a 5-month remission. Thus, drug profiling captures disease-relevant features and unexpected sensitivity to relevant drugs, which warrants further exploration of this functional assay in the context of clinical trials to develop drug repurposing strategies for patients with urgent medical needs.
© 2017 by The American Society of Hematology.
Figures



References
-
- Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120(14):2807-2816. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

