The intrinsically liganded cyclic nucleotide-binding homology domain promotes KCNH channel activation
- PMID: 28122815
- PMCID: PMC5299623
- DOI: 10.1085/jgp.201611701
The intrinsically liganded cyclic nucleotide-binding homology domain promotes KCNH channel activation
Abstract
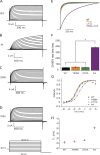
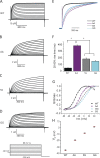
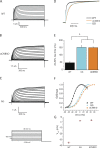
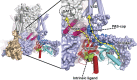
Channels in the ether-à-go-go or KCNH family of potassium channels are characterized by a conserved, C-terminal domain with homology to cyclic nucleotide-binding homology domains (CNBhDs). Instead of cyclic nucleotides, two amino acid residues, Y699 and L701, occupy the binding pocket, forming an "intrinsic ligand." The role of the CNBhD in KCNH channel gating is still unclear, however, and a detailed characterization of the intrinsic ligand is lacking. In this study, we show that mutating both Y699 and L701 to alanine, serine, aspartate, or glycine impairs human EAG1 channel function. These mutants slow channel activation and shift the conductance-voltage (G-V) relation to more depolarized potentials. The mutations affect activation and the G-V relation progressively, indicating that the gating machinery is sensitive to multiple conformations of the CNBhD. Substitution with glycine at both sites (GG), which eliminates the side chains that interact with the binding pocket, also reduces the ability of voltage prepulses to populate more preactivated states along the activation pathway (i.e., the Cole-Moore effect), as if stabilizing the voltage sensor in deep resting states. Notably, deletion of the entire CNBhD (577-708, ΔCNBhD) phenocopies the GG mutant, suggesting that GG is a loss-of-function mutation and the CNBhD requires an intrinsic ligand to exert its functional effects. We developed a kinetic model for both wild-type and ΔCNBhD mutant channels that describes all our observations on activation kinetics, the Cole-Moore shift, and G-V relations. These findings support a model in which the CNBhD both promotes voltage sensor activation and stabilizes the open pore. The intrinsic ligand is critical for these functional effects.
© 2017 Zhao et al.
Figures




References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

