Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation
- PMID: 28122965
- PMCID: PMC5322216
- DOI: 10.4049/jimmunol.1601199
Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation
Abstract
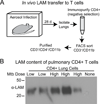
Mycobacterium tuberculosis utilizes multiple mechanisms to evade host immune responses, and inhibition of effector CD4+ T cell responses by M. tuberculosis may contribute to immune evasion. TCR signaling is inhibited by M. tuberculosis cell envelope lipoglycans, such as lipoarabinomannan and lipomannan, but a mechanism for lipoglycans to traffic from M. tuberculosis within infected macrophages to reach T cells is unknown. In these studies, we found that membrane vesicles produced by M. tuberculosis and released from infected macrophages inhibited the activation of CD4+ T cells, as indicated by reduced production of IL-2 and reduced T cell proliferation. Flow cytometry and Western blot demonstrated that lipoglycans from M. tuberculosis-derived bacterial vesicles (BVs) are transferred to T cells, where they inhibit T cell responses. Stimulation of CD4+ T cells in the presence of BVs induced expression of GRAIL, a marker of T cell anergy; upon restimulation, these T cells showed reduced ability to proliferate, confirming a state of T cell anergy. Furthermore, lipoarabinomannan was associated with T cells after their incubation with infected macrophages in vitro and when T cells were isolated from lungs of M. tuberculosis-infected mice, confirming the occurrence of lipoarabinomannan trafficking to T cells in vivo. These studies demonstrate a novel mechanism for the direct regulation of CD4+ T cells by M. tuberculosis lipoglycans conveyed by BVs that are produced by M. tuberculosis and released from infected macrophages. These lipoglycans are transferred to T cells to inhibit T cell responses, providing a mechanism that may promote immune evasion.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures
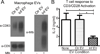





References
-
- Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb B, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect. Immun. 2010;78:5116–5125. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

