Innate Immune Responses of Bat and Human Cells to Filoviruses: Commonalities and Distinctions
- PMID: 28122983
- PMCID: PMC5375674
- DOI: 10.1128/JVI.02471-16
Innate Immune Responses of Bat and Human Cells to Filoviruses: Commonalities and Distinctions
Abstract
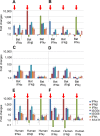
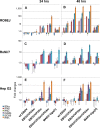
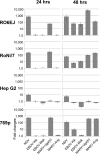
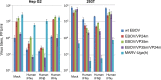
Marburg (MARV) and Ebola (EBOV) viruses are zoonotic pathogens that cause severe hemorrhagic fever in humans. The natural reservoir of MARV is the Egyptian rousette bat (Rousettus aegyptiacus); that of EBOV is unknown but believed to be another bat species. The Egyptian rousette develops subclinical productive infection with MARV but is refractory to EBOV. Interaction of filoviruses with hosts is greatly affected by the viral interferon (IFN)-inhibiting domains (IID). Our study was aimed at characterization of innate immune responses to filoviruses and the role of filovirus IID in bat and human cells. The study demonstrated that EBOV and MARV replicate to similar levels in all tested cell lines, indicating that permissiveness for EBOV at cell and organism levels do not necessarily correlate. Filoviruses, particularly MARV, induced a potent innate immune response in rousette cells, which was generally stronger than that in human cells. Both EBOV VP35 and VP24 IID were found to suppress the innate immune response in rousette cells, but only VP35 IID appeared to promote virus replication. Along with IFN-α and IFN-β, IFN-γ was demonstrated to control filovirus infection in bat cells but not in human cells, suggesting host species specificity of the antiviral effect. The antiviral effects of bat IFNs appeared not to correlate with induction of IFN-stimulated genes 54 and 56, which were detected in human cells ectopically expressing bat IFN-α and IFN-β. As bat IFN-γ induced the type I IFN pathway, its antiviral effect is likely to be partially induced via cross talk.IMPORTANCE Bats serve as reservoirs for multiple emerging viruses, including filoviruses, henipaviruses, lyssaviruses, and zoonotic coronaviruses. Although there is no evidence for symptomatic disease caused by either Marburg or Ebola viruses in bats, spillover of these viruses into human populations causes deadly outbreaks. The reason for the lack of symptomatic disease in bats infected with filoviruses remains unknown. The outcome of a virus-host interaction depends on the ability of the host immune system to suppress viral replication and the ability of a virus to counteract the host defenses. Our study is a comparative analysis of the host innate immune response to either MARV or EBOV infection in bat and human cells and the role of viral interferon-inhibiting domains in the host innate immune responses. The data are useful for understanding the interactions of filoviruses with natural and accidental hosts and for identification of factors that influence filovirus evolution.
Keywords: Ebola virus; Marburg virus; accidental host; bat; immune evasion; interferon-inhibiting domain; interferons; natural host.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
Impact of Měnglà Virus Proteins on Human and Bat Innate Immune Pathways.J Virol. 2020 Jun 16;94(13):e00191-20. doi: 10.1128/JVI.00191-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295912 Free PMC article.
-
Rousette Bat Dendritic Cells Overcome Marburg Virus-Mediated Antiviral Responses by Upregulation of Interferon-Related Genes While Downregulating Proinflammatory Disease Mediators.mSphere. 2019 Dec 4;4(6):e00728-19. doi: 10.1128/mSphere.00728-19. mSphere. 2019. PMID: 31801842 Free PMC article.
-
Marburg and Kasokero viruses elicit differential antiviral innate immune control by their natural reservoir bat, the Egyptian rousette (Rousettus aegyptiacus).Antiviral Res. 2025 Aug;240:106211. doi: 10.1016/j.antiviral.2025.106211. Epub 2025 Jun 8. Antiviral Res. 2025. PMID: 40494484
-
Evasion of interferon responses by Ebola and Marburg viruses.J Interferon Cytokine Res. 2009 Sep;29(9):511-20. doi: 10.1089/jir.2009.0076. J Interferon Cytokine Res. 2009. PMID: 19694547 Free PMC article. Review.
-
Innate immune evasion by filoviruses.Virology. 2015 May;479-480:122-30. doi: 10.1016/j.virol.2015.03.030. Epub 2015 Apr 3. Virology. 2015. PMID: 25843618 Free PMC article. Review.
Cited by
-
Marburg Virus Viral Protein 35 Inhibits Protein Kinase R Activation in a Cell Type-Specific Manner.J Infect Dis. 2018 Nov 22;218(suppl_5):S403-S408. doi: 10.1093/infdis/jiy473. J Infect Dis. 2018. PMID: 30165526 Free PMC article.
-
Comparison of antiviral responses in two bat species reveals conserved and divergent innate immune pathways.iScience. 2023 Jul 20;26(8):107435. doi: 10.1016/j.isci.2023.107435. eCollection 2023 Aug 18. iScience. 2023. PMID: 37575178 Free PMC article.
-
Tolerance and Persistence of Ebola Virus in Primary Cells from Mops condylurus, a Potential Ebola Virus Reservoir.Viruses. 2021 Oct 29;13(11):2186. doi: 10.3390/v13112186. Viruses. 2021. PMID: 34834992 Free PMC article.
-
Effects of Overexpression of the Egyptian Fruit Bat Innate Immune Genes on Filovirus Infections in the Host Cells.Front Virol. 2021 Oct;1:759655. doi: 10.3389/fviro.2021.759655. Epub 2021 Oct 12. Front Virol. 2021. PMID: 36237518 Free PMC article.
-
Host Transcriptional Response to Ebola Virus Infection.Vaccines (Basel). 2017 Sep 20;5(3):30. doi: 10.3390/vaccines5030030. Vaccines (Basel). 2017. PMID: 28930167 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

