Spontaneous dynamics of neural networks in deep layers of prefrontal cortex
- PMID: 28123005
- PMCID: PMC5376607
- DOI: 10.1152/jn.00295.2016
Spontaneous dynamics of neural networks in deep layers of prefrontal cortex
Abstract
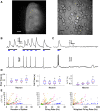
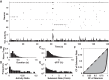
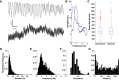
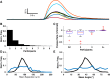
Cortical systems maintain and process information through the sustained activation of recurrent local networks of neurons. Layer 5 is known to have a major role in generating the recurrent activation associated with these functions, but relatively little is known about its intrinsic dynamics at the mesoscopic level of large numbers of neighboring neurons. Using calcium imaging, we measured the spontaneous activity of networks of deep-layer medial prefrontal cortical neurons in an acute slice model. Inferring the simultaneous activity of tens of neighboring neurons, we found that while the majority showed only sporadic activity, a subset of neurons engaged in sustained delta frequency rhythmic activity. Spontaneous activity under baseline conditions was weakly correlated between pairs of neurons, and rhythmic neurons showed little coherence in their oscillations. However, we consistently observed brief bouts of highly synchronous activity that must be attributed to network activity. NMDA-mediated stimulation enhanced rhythmicity, synchrony, and correlation within these local networks. These results characterize spontaneous prefrontal activity at a previously unexplored spatiotemporal scale and suggest that medial prefrontal cortex can act as an intrinsic generator of delta oscillations.NEW & NOTEWORTHY Using calcium imaging and a novel analytic framework, we characterized the spontaneous and NMDA-evoked activity of layer 5 prefrontal cortex at a largely unexplored spatiotemporal scale. Our results suggest that the mPFC microcircuitry is capable of intrinsically generating delta oscillations and sustaining synchronized network activity that is potentially relevant for understanding its contribution to cognitive processes.
Keywords: calcium imaging; delta oscillations; prefrontal cortex; synchrony.
Copyright © 2017 the American Physiological Society.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

