Differential Encoding of Time by Prefrontal and Striatal Network Dynamics
- PMID: 28123021
- PMCID: PMC5296780
- DOI: 10.1523/JNEUROSCI.1789-16.2016
Differential Encoding of Time by Prefrontal and Striatal Network Dynamics
Abstract
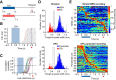
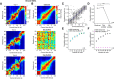
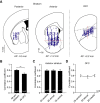
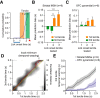
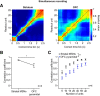
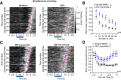
Telling time is fundamental to many forms of learning and behavior, including the anticipation of rewarding events. Although the neural mechanisms underlying timing remain unknown, computational models have proposed that the brain represents time in the dynamics of neural networks. Consistent with this hypothesis, changing patterns of neural activity dynamically in a number of brain areas-including the striatum and cortex-has been shown to encode elapsed time. To date, however, no studies have explicitly quantified and contrasted how well different areas encode time by recording large numbers of units simultaneously from more than one area. Here, we performed large-scale extracellular recordings in the striatum and orbitofrontal cortex of mice that learned the temporal relationship between a stimulus and a reward and reported their response with anticipatory licking. We used a machine-learning algorithm to quantify how well populations of neurons encoded elapsed time from stimulus onset. Both the striatal and cortical networks encoded time, but the striatal network outperformed the orbitofrontal cortex, a finding replicated both in simultaneously and nonsimultaneously recorded corticostriatal datasets. The striatal network was also more reliable in predicting when the animals would lick up to ∼1 s before the actual lick occurred. Our results are consistent with the hypothesis that temporal information is encoded in a widely distributed manner throughout multiple brain areas, but that the striatum may have a privileged role in timing because it has a more accurate "clock" as it integrates information across multiple cortical areas.
Significance statement: The neural representation of time is thought to be distributed across multiple functionally specialized brain structures, including the striatum and cortex. However, until now, the neural code for time has not been compared quantitatively between these areas. Here, we performed large-scale recordings in the striatum and orbitofrontal cortex of mice trained on a stimulus-reward association task involving a delay period and used a machine-learning algorithm to quantify how well populations of simultaneously recorded neurons encoded elapsed time from stimulus onset. We found that, although both areas encoded time, the striatum consistently outperformed the orbitofrontal cortex. These results suggest that the striatum may refine the code for time by integrating information from multiple inputs.
Keywords: decoding; machine-learning algorithm; neural dynamics; orbitofrontal cortex; striatum; time coding.
Copyright © 2017 the authors 0270-6474/17/370854-17$15.00/0.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
