Abnormal neurogenesis and cortical growth in congenital heart disease
- PMID: 28123074
- PMCID: PMC5467873
- DOI: 10.1126/scitranslmed.aah7029
Abnormal neurogenesis and cortical growth in congenital heart disease
Abstract
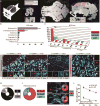
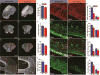
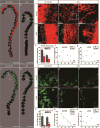
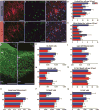
Long-term neurological deficits due to immature cortical development are emerging as a major challenge in congenital heart disease (CHD). However, cellular mechanisms underlying dysregulation of perinatal corticogenesis in CHD remain elusive. The subventricular zone (SVZ) represents the largest postnatal niche of neural stem/progenitor cells (NSPCs). We show that the piglet SVZ resembles its human counterpart and displays robust postnatal neurogenesis. We present evidence that SVZ NSPCs migrate to the frontal cortex and differentiate into interneurons in a region-specific manner. Hypoxic exposure of the gyrencephalic piglet brain recapitulates CHD-induced impaired cortical development. Hypoxia reduces proliferation and neurogenesis in the SVZ, which is accompanied by reduced cortical growth. We demonstrate a similar reduction in neuroblasts within the SVZ of human infants born with CHD. Our findings demonstrate that SVZ NSPCs contribute to perinatal corticogenesis and suggest that restoration of SVZ NSPCs' neurogenic potential is a candidate therapeutic target for improving cortical growth in CHD.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures




References
-
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. The New England journal of medicine. 2005;353:811–822. - PubMed
-
- Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

