RON kinase: A target for treatment of cancer-induced bone destruction and osteoporosis
- PMID: 28123075
- PMCID: PMC5771677
- DOI: 10.1126/scitranslmed.aai9338
RON kinase: A target for treatment of cancer-induced bone destruction and osteoporosis
Abstract
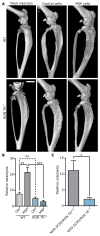
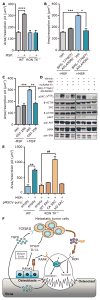
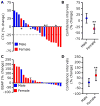
Bone destruction occurs in aging and numerous diseases, including osteoporosis and cancer. Many cancer patients have bone osteolysis that is refractory to state-of-the-art treatments, which block osteoclast activity with bisphosphonates or by inhibiting the receptor activator of nuclear factor κB ligand (RANKL) pathway. We previously showed that macrophage-stimulating protein (MSP) signaling, which is elevated in about 40% of breast cancers, promotes osteolytic bone metastasis by activation of the MSP signaling pathway in tumor cells or in the bone microenvironment. We show that MSP signals through its receptor, RON tyrosine kinase, expressed on host cells, to activate osteoclasts directly by a previously undescribed pathway that is complementary to RANKL signaling and converges on proto-oncogene, non-receptor tyrosine kinase SRC (SRC). Genetic or pharmacologic inhibition of RON kinase blocked cancer-mediated bone destruction and osteoporosis in several mouse models. Furthermore, the RON kinase inhibitor BMS-777607/ASLAN002 altered markers of bone turnover in a first-in-human clinical cancer study, indicating the inhibitor's potential for normalizing bone loss in patients. These findings uncover a new therapeutic target for pathogenic bone loss and provide a rationale for treatment of bone destruction in various diseases with RON inhibitors.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures



References
-
- National Osteoporosis Foundation report. 2002
-
- Coleman RE. Future directions in the treatment and prevention of bone metastases. Am J Clin Oncol. 2002;25:S32–38. - PubMed
-
- Diel IJ, Solomayer EF, Bastert G. Treatment of metastatic bone disease in breast cancer: bisphosphonates. Clin Breast Cancer. 2000;1:43–51. - PubMed
-
- Melton LJ, 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20:487–493. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

