Role of free testosterone levels in patients with metastatic castration-resistant prostate cancer receiving second-line therapy
- PMID: 28123517
- PMCID: PMC5244876
- DOI: 10.3892/ol.2016.5392
Role of free testosterone levels in patients with metastatic castration-resistant prostate cancer receiving second-line therapy
Abstract
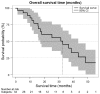
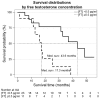
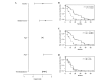
A range of new treatment options has recently become available for patients with advanced metastatic castration-resistant prostate cancer (mCRPC). Androgen deprivation therapy (ADT) with luteinizing hormone-releasing hormone is continued when performing chemotherapy or androgen deprivation with new second-generation therapeutic agents such as enzalutamide or abiraterone acetate. Despite the fact that free testosterone (FT) is the biologically active form, it is common practice that androgen suppression is monitored via total testosterone levels only. The aim of the present study was to evaluate the role of FT as a prognostic biomarker for cancer-specific survival (CSS) and its feasibility as an ADT monitoring biomarker in patients with mCRPC for the first time. The requirement for continued ADT in mCRPC patients is discussed within the basis of the current literature. A total of 34 patients with continuous measurements of FT levels and mCRPC status underwent therapy with docetaxel, abiraterone acetate, enzalutamide, cabozantinib, carboplatin or cabazitaxel. Data were obtained from the Departments of Urology and Urological Oncology, Hannover Medical School (Hannover, Germany) between March 2009 and April 2014. A cutoff point of 0.5 pg/ml was used to discriminate between patients according to FT levels. Statistical evaluation of CSS was performed by applying Kaplan Meier survival estimates, multivariate Cox regression analyses and log-rank tests. The median age of all 34 patients was 72 years (range, 51-86 years). The mean follow-up interval was 16.1 months (range, 0.7-55.6 months). Despite the fact that all patients were undergoing androgen deprivation, the mean serum FT levels for each patient varied; the mean FT concentration in the cohort was 0.328 pg/ml, ranging from 0.01-9.1 pg/ml. A notable difference with regard to CSS was observed for patients with regard to serum FT concentration; CSS was significantly longer for patients with a serum FT level below the cutoff level (43.6 vs. 17.3 months, respectively, P=0.0063). Upon multivariate Cox regression analysis, the mean FT concentration during treatment remained a significant prognostic factor for CSS (hazard ratio, 1.22; 95% confidence interval, 1.03-1.43; P=0.0182). In conclusion, in patients with mCRPC, the serum FT level is a strong predictor of CSS in patients under therapy with second-line anti-hormonal therapeutic medication and chemotherapy. It may be concluded that FT levels should be included into the routine control of androgen suppression while under treatment with ADT and second-generation hormonal therapy.
Keywords: abiraterone acetate; androgen deprivation therapy; enzalutamide; free testosterone; luteinizing hormone-releasing hormone; metastatic castration-resistant prostate cancer.
Figures

References
-
- Huggins C, Stevens RE, Hodges CV. Studies on prostate cancer. II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. doi: 10.1001/archsurg.1941.01210140043004. - DOI
-
- Walsh PC. Physiologic basis for hormonal therapy in carcinoma of the prostate. Urol Clin North Am. 1975;2:125–140. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
