Racecadotril for the treatment of severe acute watery diarrhoea in children admitted to a tertiary hospital in Kenya
- PMID: 28123772
- PMCID: PMC5253457
- DOI: 10.1136/bmjgast-2016-000124
Racecadotril for the treatment of severe acute watery diarrhoea in children admitted to a tertiary hospital in Kenya
Abstract
Background: Diarrhoea is the second most common cause of death in children under 5 years of age in Kenya. It is usually treated with oral rehydration, zinc and continued feeding. Racecadotril has been in use for over 2 decades; however, there is a paucity of data regarding its efficacy from Africa.
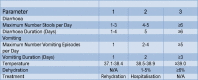
Objectives: The objectives of this study were: to compare the number of stools in the first 48 hours in children with severe gastroenteritis requiring admission and treated with either racecadotril or placebo, to study the impact of racecadotril on duration of inpatient stay as well as duration of diarrhoea and to describe the side effect profile of racecadotril.
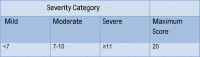
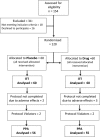
Methods: This was a randomised, double-blinded, placebo-controlled trial. It enrolled children between the age of 3 and 60 months who were admitted with severe acute gastroenteritis. They received either racecadotril or placebo in addition to oral rehydration solution (ORS) and zinc and were followed up daily.
Results: 120 children were enrolled into the study. There were no differences in the demographics or outcomes between the 2 groups. Stools at 48 hours: median (IQR) of 5 (3-7) and 5 (2.5-7.5), respectively; p=0.63. The duration of inpatient stay: median (IQR): 4 days (1.5-6.5) and 4.5 (1.8-6.3); p=0.71. The duration of illness: 3 days (2-4) and 2 days (1-3); p=0.77. The relative risk of a severe adverse event was 3-fold higher in the drug group but was not statistically significant (95% CI 0.63 to 14.7); p=0.16.
Conclusions: Racecadotril has no impact on the number of stools at 48 hours, the duration of hospital stay or the duration of diarrhoea in children admitted with severe gastroenteritis and managed with ORS and zinc.
Trial registration number: PACTR201403000694398; Pre-results.
Keywords: ADJUVANT TREATMENT; CLINICAL TRIALS; INFECTIOUS DIARRHOEA; INTESTINAL SECRETION; PAEDIATRIC DIARRHOEA.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Organisation WH. Global burden of disease estimates. Geneva: World Health Organisation, 2007.
-
- Onyango D, Angienda P. Epidemiology of waterborne diarrheal diseases among children aged 6–36 months old in Busia—Western Kenya. Int Sci Index 2010;4:1001–8.
-
- UNICEF. Statistics by topic. Child health. Diarrhoeal disease. Current status and progress. (Updated June 2016). http://data.unicef.org/child-health/diarrhoeal-disease.html (accessed 8 Aug 2016)
-
- Matheson AL, Noble S. Racecadotril. Drugs 2000;59:829–35. doi:10.2165/00003495-200059040-00010 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
