Identifying a core set of outcome domains to measure in clinical trials for shoulder disorders: a modified Delphi study
- PMID: 28123775
- PMCID: PMC5237745
- DOI: 10.1136/rmdopen-2016-000380
Identifying a core set of outcome domains to measure in clinical trials for shoulder disorders: a modified Delphi study
Abstract
Objective: To achieve consensus on the most important outcome domains to measure across all clinical trials for shoulder disorders.
Methods: We performed an online modified Delphi study with an international, multidisciplinary and multistakeholder panel. A literature review and the OMERACT Filter 2.0 framework was used to generate a list of potential core domains, which were presented to patients, clinicians and researchers in two Delphi rounds. Participants were asked to judge the importance of each potential core domain and provide a rationale for their response. A core domain was defined a priori as a domain that at least 67% of participants considered core.
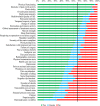
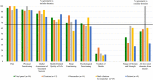
Results: In both rounds, 335 individuals were invited to participate (268 clinicians/researchers and 67 patients); response rates were 27% (n=91) and 29% (n=96), respectively. From a list of 41 potential core domains, four domains met our criteria for inclusion: 'pain', 'physical functioning', 'global assessment of treatment success' and 'health-related quality of life'. Two additional domains, 'sleep functioning' and 'psychological functioning', met the criteria for inclusion by some, but not all stakeholder groups. There was consensus that 'number of deaths' was not a core domain, but insufficient agreement on whether or not several other domains, including 'range of motion' and 'muscle strength', were core domains.
Conclusions: Based on international consensus from patients, clinicians and researchers, 'pain', 'physical functioning', 'global assessment of treatment success' and 'health-related quality of life' were considered core outcome domains for shoulder disorder trials. The value of several other domains needs further consideration.
Keywords: Outcomes research; Patient perspective; Qualitative research.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
