Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages
- PMID: 28123869
- PMCID: PMC5213309
- DOI: 10.1080/2162402X.2016.1229725
Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages
Abstract
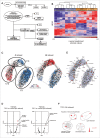
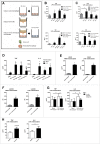
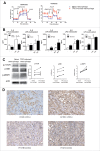
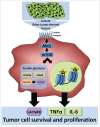
Tumor-associated macrophages (TAMs) are key components of the tumor microenvironment in non-medullary thyroid cancer (TC), the most common endocrine malignancy. However, little is known regarding the regulation of their function in TC. Transcriptome analysis in a model of TC-induced macrophages identified increased inflammatory characteristics and rewiring of cell metabolism as key functional changes. This functional reprogramming was partly mediated by TC-derived lactate that induced upregulation of cytokine production through an AKT1/mTOR-dependent increase in aerobic glycolysis. This led to epigenetic modifications at the level of histone methylation, and subsequently long-term functional changes. Immunohistochemistry assessment validated the increase in glycolysis enzymes and lactate receptor in TAMs in tissue samples from patients with TC. In conclusion, Akt/mTOR-dependent glycolysis mediates TC-induced reprogramming of TAMs and inflammation, and this may represent a novel therapeutic target in TC.
Keywords: Cytokines; epigenetics; immunometabolism; lactate; thyroid cancer; tumor-associated macrophages.
Figures

References
-
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/10.1038/nature07205 - DOI - PubMed
-
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41:49-61; PMID:25035953; http://dx.doi.org/10.1016/j.immuni.2014.06.010 - DOI - PMC - PubMed
-
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM et al.. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014; 513:559-63; PMID:25043024; http://dx.doi.org/10.1038/nature13490 - DOI - PMC - PubMed
-
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig 2012; 122:787-95; PMID:22378047; http://dx.doi.org/10.1172/JCI59643 - DOI - PMC - PubMed
-
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014; 140:317-22; PMID:24557566; http://dx.doi.org/10.1001/jamaoto.2014.1 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
