Identification of a tumor-reactive T-cell repertoire in the immune infiltrate of patients with resectable pancreatic ductal adenocarcinoma
- PMID: 28123878
- PMCID: PMC5215250
- DOI: 10.1080/2162402X.2016.1240859
Identification of a tumor-reactive T-cell repertoire in the immune infiltrate of patients with resectable pancreatic ductal adenocarcinoma
Abstract
Purpose: The devastating prognosis of patients with resectable pancreatic ductal adenocarcinoma (PDA) presents an urgent need for the development of therapeutic strategies targeting disseminated tumor cells. Until now, T-cell therapy has been scarcely pursued in PDA, due to the prevailing view that it represents a poorly immunogenic tumor.
Experimental design: We systematically analyzed T-cell infiltrates in tumor biopsies from 127 patients with resectable PDA by means of immunohistochemistry, flow cytometry, T-cell receptor (TCR) deep-sequencing and functional analysis of in vitro expanded T-cell cultures. Parallel studies were performed on tumor-infiltrating lymphocytes (TIL) from 44 patients with metastatic melanoma.
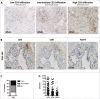
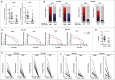
Results: Prominent T-cell infiltrates, as well as tertiary lymphoid structures harboring proliferating T-cells, were detected in the vast majority of biopsies from PDA patients. The notion that the tumor is a site of local T-cell expansion was strengthened by TCR deep-sequencing, revealing that the T-cell repertoire in the tumor is dominated by highly frequent CDR3 sequences that can be up to 10,000-fold enriched in tumor as compared to peripheral blood. In fact, TCR repertoire composition in PDA resembled that in melanoma. Moreover, in vitro expansion of TILs was equally efficient for PDA and melanoma, resulting in T-cell cultures displaying HLA class I-restricted reactivity against autologous tumor cells.
Conclusions: The tumor-infiltrating T-cell response in PDA shows striking similarity to that in melanoma, where adoptive T-cell therapy has significant therapeutic impact. Our findings indicate that T-cell-based therapies may be used to counter disease recurrence in patients with resectable PDA.
Keywords: Adoptive T-cell therapy; T-cell receptor (TCR) repertoire; pancreatic ductal adenocarcinoma; tertiary lymphoid structures; tumor-infiltrating lymphocytes.
Figures




References
-
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015; 161:205-14; PMID:25860605; http://dx.doi.org/10.1016/j.cell.2015.03.030 - DOI - PMC - PubMed
-
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450-61; PMID:25858804; http://dx.doi.org/10.1016/j.ccell.2015.03.001 - DOI - PMC - PubMed
-
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010; 7:e1000267; PMID:20422030; http://dx.doi.org/10.1371/journal.pmed.1000267 - DOI - PMC - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C et al.. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817-25; PMID:21561347; http://dx.doi.org/10.1056/NEJMoa1011923 - DOI - PubMed
-
- Hartwig W, Werner J, Jager D, Debus J, Buchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol 2013; 14:e476-85; PMID:24079875; http://dx.doi.org/10.1016/S1470-2045(13)70172-4 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
