The non-inflammatory role of C1q during Her2/neu-driven mammary carcinogenesis
- PMID: 28123895
- PMCID: PMC5214935
- DOI: 10.1080/2162402X.2016.1253653
The non-inflammatory role of C1q during Her2/neu-driven mammary carcinogenesis
Abstract
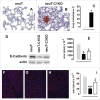
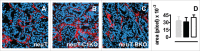
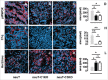
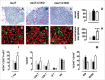
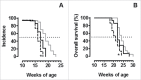
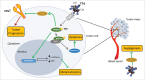
There is an ever increasing amount of evidence to support the hypothesis that complement C1q, the first component of the classical complement pathway, is involved in the regulation of cancer growth, in addition to its role in fighting infections. It has been demonstrated that C1q is expressed in the microenvironment of various types of human tumors, including breast adenocarcinomas. This study compares carcinogenesis progression in C1q deficient (neuT-C1KO) and C1q competent neuT mice in order to investigate the role of C1q in mammary carcinogenesis. Significantly accelerated autochthonous neu+ carcinoma progression was paralleled by accelerated spontaneous lung metastases occurrence in C1q deficient mice. Surprisingly, this effect was not caused by differences in the tumor-infiltrating cells or in the activation of the complement classical pathway, since neuT-C1KO mice did not display a reduction in C3 fragment deposition at the tumor site. By contrast, a significant higher number of intratumor blood vessels and a decrease in the activation of the tumor suppressor WW domain containing oxidoreductase (WWOX) were observed in tumors from neuT-C1KO as compare with neuT mice. In parallel, an increase in Her2/neu expression was observed on the membrane of tumor cells. Taken together, our findings suggest that C1q plays a direct role both on halting tumor angiogenesis and on inducing apoptosis in mammary cancer cells by coordinating the signal transduction pathways linked to WWOX and, furthermore, highlight the role of C1q in mammary tumor immune surveillance regardless of complement system activation.
Keywords: C1q; Complement; ErbB2; Her2/neu; genetically engineered mice; immunosurveillance; mammary cancer.
Figures

References
-
- Mamidi S, Höne S, Kirschfink M. The complement system in cancer: Ambivalence between tumour destruction and promotion. Immunobiology 2015; 222(1):45-54; PMID:26686908; http://dx.doi.org/10.1016/j.imbio.2015.11.008 - DOI - PubMed
-
- Stover C. Dual role of complement in tumour growth and metastasis (Review). Int J Mol Med 2010; 25:307-13; PMID:20127033; http://dx.doi.org/10.3892/ijmm_00000346 - DOI - PubMed
-
- Pio R, Corrales L, Lambris JD. The role of complement in tumor growth. Adv Exp Med Biol 2014; 772:229-62. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4379038&to... PMID:24272362; http://dx.doi.org/10.1007/978-1-4614-5915-6_11 - DOI - PMC - PubMed
-
- Markiewski MM, Lambris JD. Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol 2009; 30:286-92. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid =2704572&tool=pmcentrez&rendertype=abstract; PMID:19428302; http://dx.doi.org/10.1016/j.it.2009.04.002 - DOI - PMC - PubMed
-
- Cooper PD. Complement and cancer: activation of the alternative pathway as a theoretical base for immunotherapy. Adv Immun Cancer Ther 1985; 1:125-66; PMID:3916662; http://dx.doi.org/10.1007/978-1-4612-5068-5_4 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
