Fetal development of subcutaneous white adipose tissue is dependent on Zfp423
- PMID: 28123942
- PMCID: PMC5220400
- DOI: 10.1016/j.molmet.2016.11.009
Fetal development of subcutaneous white adipose tissue is dependent on Zfp423
Abstract
Objective: Zfp423 is a multi zinc-finger transcription factor expressed in preadipocytes and mature adipocytes in vivo. Our recent work has revealed a critical role for Zfp423 in maintaining the fate of white adipocytes in adult mice through suppression of the beige cell thermogenic gene program; loss of Zfp423 in mature adipocytes of adult mice results in a white-to-beige phenotypic switch. However, the exact requirements of Zfp423 in the fetal stages of early adipose development in vivo have not been clarified.
Method: Here, we utilize two models that confer adipose-specific Zfp423 inactivation during fetal adipose development (Adiponectin-Cre; Zfp423loxP/loxP and Adiponectin-rtTA; TRE-Cre; Zfp423loxP/loxP). We assess the impact of fetal adipose Zfp423 deletion on the initial formation of adipose tissue and evaluate the metabolic consequences of challenging these animals with high-fat diet feeding.
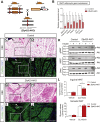
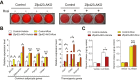
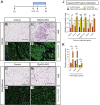
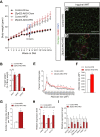
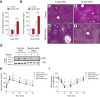
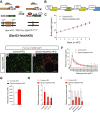
Results: Deletion of Zfp423 during fetal adipose development results in a different phenotype than is observed when deleting Zfp423 in adipocytes of adult mice. Inactivation of Zfp423 during fetal adipose development results in arrested differentiation, specifically of inguinal white adipocytes, rather than a white-to-beige phenotypic switch that occurs when Zfp423 is inactivated in adult mice. This is likely explained by the observation that adiponectin driven Cre expression is active at an earlier stage of the adipocyte life cycle during fetal subcutaneous adipose development than in adult mice. Upon high-fat diet feeding, obese adipose Zfp423-deficient animals undergo a pathological adipose tissue expansion, associated with ectopic lipid deposition and systemic insulin resistance.
Conclusions: Our results reveal that Zfp423 is essential for the terminal differentiation of subcutaneous white adipocytes during fetal adipose tissue development. Moreover, our data highlight the striking adverse effects of pathological subcutaneous adipose tissue remodeling on visceral adipose function and systemic nutrient homeostasis in obesity. Importantly, these data reveal the distinct phenotypes that can occur when adiponectin driven transgenes are activated in fetal vs. adult adipose tissue.
Keywords: Adipogenesis; Insulin resistance; Obesity; Pparg; Preadipocytes; Subcutaneous adipocytes; Zfp423.
Figures

References
-
- Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013;19(10):1252–1263. - PubMed
-
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Review. 2004;84(1):277–359. - PubMed
-
- Unger R.H., Clark G.O., Scherer P.E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochimica et Biophysica Acta. 2010;1801(3):209–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

