Minimally invasive treatments for venous compression syndromes
- PMID: 28123978
- PMCID: PMC5220193
- DOI: 10.21037/cdt.2016.10.01
Minimally invasive treatments for venous compression syndromes
Abstract
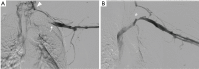
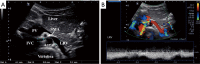
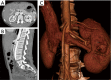
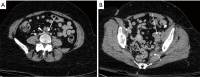
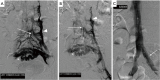
The management of venous compression syndromes has historically been reliant on surgical treatment when conservative measures fail. There are, however, several settings in which endovascular therapy can play a significant role as an adjunct or even a replacement to more invasive surgical methods. We explore the role of minimally invasive treatment options for three of the most well-studied venous compression syndromes. The clinical aspects and pathophysiology of Paget-Schroetter syndrome (PSS), nutcracker syndrome, and May-Thurner syndrome are discussed in detail, with particular emphasis on the role that interventionalists can play in minimally invasive treatment.
Keywords: Endovascular procedures; May-Thurner syndrome; renal nutcracker syndrome; thrombolytic therapy; upper extremity deep vein thrombosis (UEDVT).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
