Global Assessment of the Activity of Tigecycline against Multidrug-Resistant Gram-Negative Pathogens between 2004 and 2014 as Part of the Tigecycline Evaluation and Surveillance Trial
- PMID: 28124025
- PMCID: PMC5244261
- DOI: 10.1128/mSphere.00310-16
Global Assessment of the Activity of Tigecycline against Multidrug-Resistant Gram-Negative Pathogens between 2004 and 2014 as Part of the Tigecycline Evaluation and Surveillance Trial
Abstract
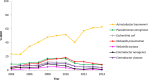
Multidrug-resistant (MDR) Gram-negative organisms are a burden on the global health care system. The Tigecycline Evaluation and Surveillance Trial (TEST) is an ongoing global study designed to monitor the in vitro activities of tigecycline and a panel of marketed antimicrobials against a range of clinically significant pathogens. In this study, in vitro data are presented for MDR Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Enterobacter aerogenes, and Enterobacter cloacae isolates collected from 2004 to 2014. In total, 13% (21,967/170,759) of isolates displayed multidrug resistance globally, with the highest rates recorded among A. baumannii (overall rate, 44% [8,294/18,741], increasing from 23% [309/1,323] in 2004 to 63% [447/712] in 2014). Other multidrug resistance rates ranged from 2.5% for K. oxytoca (203/8,000) to 12% for P. aeruginosa and K. pneumoniae (3,951/32,786 and 3,895/32,888, respectively), and rates among these pathogens remained stable during the study period. Against MDR E. coli, Klebsiella spp., and E. aerogenes, the lowest rates of resistance were to tigecycline (0.2%, 6%, and 12%, respectively), and the lowest MIC90 value against A. baumannii was observed for tigecycline (2 mg/liter; MIC range, ≤0.008 to ≥32 mg/liter). The only significant change in resistance to tigecycline during the study period was for MDR E. coli (P < 0.01), among which eight resistant isolates were identified globally from 2009 to 2013. In summary, these results show that tigecycline retained in vitro activity against the majority of MDR Gram-negative organisms presented here, but the rising rates of MDR A. baumannii highlight the need for the continued monitoring of global multidrug resistance. IMPORTANCE Multidrug resistance among bacterial pathogens is an ongoing global problem and renders antimicrobial agents ineffective at treating bacterial infections. In the health care setting, infections caused by multidrug-resistant (MDR) Gram-negative bacteria can cause increased mortality, longer hospital stays, and higher treatments costs. The aim of the Tigecycline Evaluation and Surveillance Trial (TEST) is to assess the in vitro antimicrobial activities of tigecycline and other contemporary agents against clinically relevant pathogens. This paper presents antimicrobial activity data from the TEST study between 2004 and 2014 and examines global rates of MDR Gram-negative isolates, including Acinetobacter baumannii, Pseudomonas aeruginosa, and members of the Enterobacteriaceae, during this time. Our results show that tigecycline retained in vitro activity against many MDR Gram-negative pathogens over the study period, while rates of MDR A. baumannii increased globally. Using these findings, we hope to highlight the current status of multidrug resistance in medical facilities worldwide.
Keywords: Gram-negative bacteria; multidrug resistance; surveillance studies; tigecycline.
Figures
References
-
- De Angelis GD, D’Inzeo T, Fiori B, Spanu T, Sganga G. 2014. Burden of antibiotic resistant Gram negative bacterial infections: evidence and limits. J Med Microbiol Diagn 3:132–137. doi:10.4172/2161-0703.1000132. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous