Nucleic acids as cofactors for factor XI and prekallikrein activation: Different roles for high-molecular-weight kininogen
- PMID: 28124063
- PMCID: PMC5696789
- DOI: 10.1160/TH16-09-0691
Nucleic acids as cofactors for factor XI and prekallikrein activation: Different roles for high-molecular-weight kininogen
Abstract
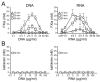
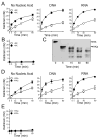
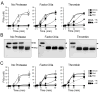
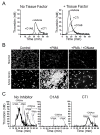
The plasma zymogens factor XI (fXI) and prekallikrein (PK) are activated by factor XIIa (fXIIa) during contact activation. Polyanions such as DNA and RNA may contribute to thrombosis and inflammation partly by enhancing PK and fXI activation. We examined PK and fXI activation in the presence of nucleic acids, and determine the effects of the cofactor high molecular weight kininogen (HK) on the reactions. In the absence of HK, DNA and RNA induced fXI autoactivation. Proteases known to activate fXI (fXIIa and thrombin) did not enhance this process appreciably. Nucleic acids had little effect on PK activation by fXIIa in the absence of HK. HK had significant but opposite effects on PK and fXI activation. HK enhanced fXIIa activation of PK in the presence of nucleic acids, but blocked fXI autoactivation. Thrombin and fXIIa could overcome the HK inhibitory effect on autoactivation, indicating these proteases are necessary for nucleic acid-induced fXI activation in an HK-rich environment such as plasma. In contrast to PK, which requires HK for optimal activation, fXI activation in the presence of nucleic acids depends on anion binding sites on the fXI molecule. The corresponding sites on PK are not necessary for PK activation. Our results indicate that HK functions as a cofactor for PK activation in the presence of nucleic acids in a manner consistent with classic models of contact activation. However, HK has, on balance, an inhibitory effect on nucleic acid-supported fXI activation and may function as a negative regulator of fXI activation.
Keywords: Contact phase; coagulation factors; proteases.
Figures




References
-
- Fischer S, Preissner KT. Extracellular nucleic acids as novel alarm signals in the vascular system. Mediators of defense and disease Hamostaseologie. 2013;33:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

